Page 113 - Tunable Lasers Handbook
P. 113
94 Charles Freed
8000
u=3
6000
r
A
k
I
1
& 4000 [I 1,03’1 I,
a
W
3 -
-
[I 220,0420],
2000
z - U=O
0
C02 GROUND STATE (00’0) N2 GROUND STATE
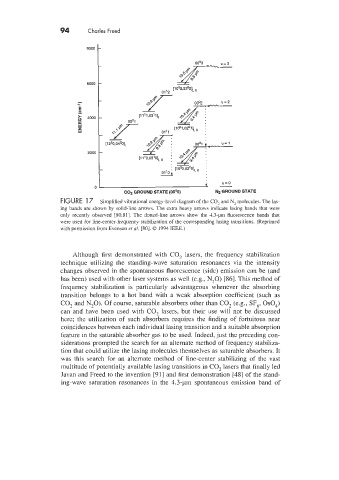
FIGURE 1 7 Simplified vibrational energy-level diagram of the CO, and N2 molecules. The las-
ing bands are shown by solid-line arrows. The extra heavy arrows indicate lasing bands that were
only recently observed [80.81]. The dotted-line arrows show the 1.3-pm fluorescence bands that
were used for line-center-frequency stabilization of the corresponding lasing transitions. (Reprinted
!&irh permission from Evenson er al. [80]. Q 1994 IEEE.)
Although first demonstrated with CO, lasers, the frequency stabilization
technique utilizing the standing-wave saturation resonances via the intensity
changes observed in the spontaneous fluorescence (side) emission can be (and
has been) used with other laser systems as well (e.g., N,O) [86]. This method of
frequency stabilization is particularly advantageous whenever the absorbing
transition belongs to a hot band with a weak absorption coefficient (such as
CO, and N,O). Of course, saturable absorbers other than CO, (e.g., SF,, OsO,)
can-and have been used with CO, lasers, but their use will not be discussed
here; the utilization of such absoibers requires the finding of fortuitous near
coincidences between each individual lasing transition and a suitable absorption
feature in the saturable absorber gas to be used. Indeed, just the preceding con-
siderations prompted the search for an alternate method of frequency stabiliza-
tion that could utilize the lasing molecules themselves as saturable absorbers. It
was this search for an alternate method of line-center stabilizing of the vast
multitude of potentially available lasing transitions in CO, lasers that finally led
Javan and Freed to the invention [91] and first demonstration [48] of the stand-
ing-wave saturation resonances in the 4.3-pm spontaneous emission band of