Page 188 - Uninterruptible Power Supplies
P. 188
Batteries
186 Chapter Seven
Chemical Reactions/Basic Design
The chemical reaction that occurs is best illustrated as follows:
1. Reaction at the negative plate (pure lead)
Pb H 2 SO 4 → PbSO 4 H 2
2. Reaction at the positive plate (lead oxide)
PbO 2 H 2 SO 4 → PbSO 4 (2OH)
Adding together 1 and 2 the chemical reaction for the cell is:
PbO 2 Pb 2H 2 SO 4 → 2PbSO 4 2H 2 O
As the action proceeds and the discharge is complete, the plates are
covered in the case of the negative plate with lead sulphate and the pos-
itive plate with a mixture of lead compounds, PbO and PbSO.
Recharging the cell involves converting the lead sulphate to lead.
Thus, the reactions are now as follows:
PbSO 4 (2H) → H 2 SO 4 Pb
The peroxide is regenerated as follows:
PbSO 4 (2OH) → PbO 2 H 2 SO 4
The complete reaction is thus expressed as:
2PbSO 4 2H 2 O → Pb PbO 2 2H 2 SO 4
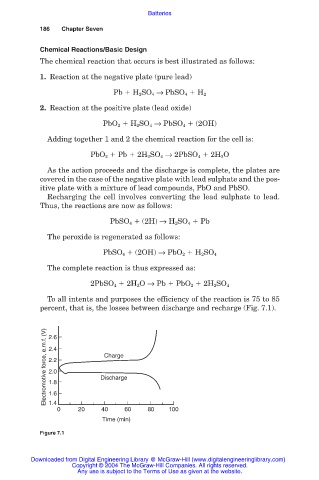
To all intents and purposes the efficiency of the reaction is 75 to 85
percent, that is, the losses between discharge and recharge (Fig. 7.1).
Electromotive force, e.m.f. (V) 2.4 Discharge
2.6
Charge
2.2
2.0
1.8
1.6
1.4
0 20 40 60 80 100
Time (min)
Figure 7.1
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.