Page 206 - Uninterruptible Power Supplies
P. 206
Batteries
204 Chapter Seven
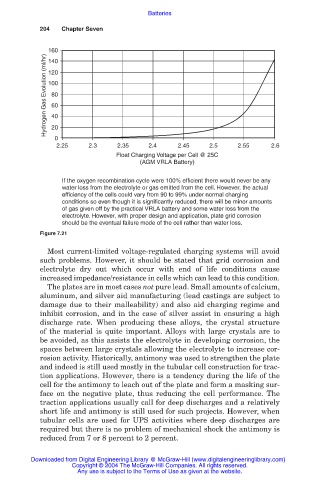
160
Hydrogen Gas Evolution (ml/hr) 100
140
120
80
60
40
20
0
2.25 2.3 2.35 2.4 2.45 2.5 2.55 2.6
Float Charging Voltage per Cell @ 25C
(AGM VRLA Battery)
If the oxygen recombination cycle were 100% efficient there would never be any
water loss from the electrolyte or gas emitted from the cell. However, the actual
efficiency of the cells could vary from 90 to 99% under normal charging
conditions so even though it is significantly reduced, there will be minor amounts
of gas given off by the practical VRLA battery and some water loss from the
electrolyte. However, with proper design and application, plate grid corrosion
should be the eventual failure mode of the cell rather than water loss.
Figure 7.21
Most current-limited voltage-regulated charging systems will avoid
such problems. However, it should be stated that grid corrosion and
electrolyte dry out which occur with end of life conditions cause
increased impedance/resistance in cells which can lead to this condition.
The plates are in most cases not pure lead. Small amounts of calcium,
aluminum, and silver aid manufacturing (lead castings are subject to
damage due to their malleability) and also aid charging regime and
inhibit corrosion, and in the case of silver assist in ensuring a high
discharge rate. When producing these alloys, the crystal structure
of the material is quite important. Alloys with large crystals are to
be avoided, as this assists the electrolyte in developing corrosion, the
spaces between large crystals allowing the electrolyte to increase cor-
rosion activity. Historically, antimony was used to strengthen the plate
and indeed is still used mostly in the tubular cell construction for trac-
tion applications. However, there is a tendency during the life of the
cell for the antimony to leach out of the plate and form a masking sur-
face on the negative plate, thus reducing the cell performance. The
traction applications usually call for deep discharges and a relatively
short life and antimony is still used for such projects. However, when
tubular cells are used for UPS activities where deep discharges are
required but there is no problem of mechanical shock the antimony is
reduced from 7 or 8 percent to 2 percent.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.