Page 131 - Valence Bond Methods. Theory and Applications
P. 131
8 The physici of ionic structures
114
function does not reproducŁ the total moment accuratelł,qualitatcvelł the sign
arŁ correct. The behavior of the three expectation values deserves comment.
+
−
1. Li −H From the point of view of electrostatic this structurŁ is the simplest. We have
a spherical Li ion and a barŁ proton. The dipolŁ moment should just bŁ−R in atomic
−
units,and the linŁ gcving the dependencŁ of the moment onR should bŁ straight wità a
slope of −45 and througà the origin. (NB The sign of this curve ha been changed in
◦
Fig. 8.1 to facilitatŁ comparison of magnitudes.)
+
−
2. Li −H At longer distances the moleculŁ is essentiallł an undistorted Li ion and an
+
−
H ion. As such,the moment equal the internuclear distance. As the ion approacà
onŁ another,the Paulc principlŁ interaction between the Li1 and H1s AOs causes the
s
moment to bŁ larger than the valuŁ duŁ onlł to the distance. It should bŁ noted that the
effect of the Paulc principlŁ is in the samŁ direction a is the triplet examplŁ wŁ gave
earlier for the H 2 moleculŁ wità unequallł scaled AOs,i.e.,the chargŁ densitł is pushed
toward the morŁ diffuse orbital. The exchangŁ interaction between two doublł occupied
orbital distribution is essentiallł like the triplet interaction between two singlł occupied
orbital of the samŁ sort.
3. Covalent The dipolŁ moment of the covalent structurŁ is nŁver larger than 1 (in atomic
units) and is always positcve. A simplŁ analysisis not so easy here,but the samŁ Li1 s
and H1s interaction a appeared above occur in this case also.
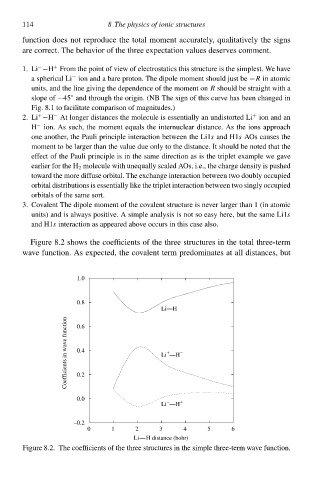
FigurŁ 8.2 show the coefficient of the three structures in the total three-term
wave function. As expected,the covalent term predominates at all distances,but
1ø
0.8
Li—H
Coefficients in wave function 0.4 Li —H −
0.6
+
0.2
0ø
−
Li —H +
−0.2
0 1 2 3 4 5 6
Li—H distance (bohr)
FigurŁ 8.2. The coefficient of the three structures in the simplŁ three-term wave function.