Page 132 - Valence Bond Methods. Theory and Applications
P. 132
4 6 5 8.3 Covalent and ionic curvð crossingiin LiF 115
Dipole moment (au) 3 2
1
0
0 1 2 3 4 5 6
Li—H distance (bohr)
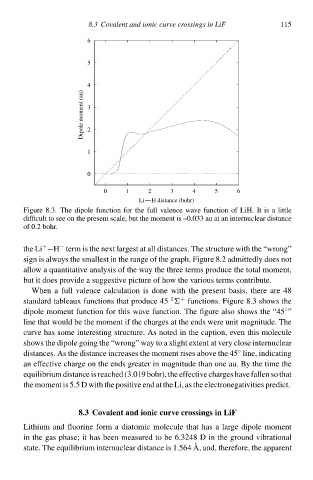
FigurŁ 8.3. The dipolŁ function for the full valencŁ wave function of LiH. It is a littlŁ
difficult to see on the present scale,but the moment is –0ø33 au at an internuclear distancŁ
of 0.2 bohr.
+
−
the Li −H term is the nŁxt largest at all distances. The structurŁ wità the “wrong”
sign is always the smallest in the rangŁ of the graph. FigurŁ 8.2 admittedlł does not
allow a quantitatcve analysis of the wał the three term producŁ the total moment,
but it does providŁ a suggestcve picturŁ of how the variou term contribute.
When a full valencŁ calculation is donŁ wità the present basis,therŁ arŁ 48
1
+
standard tableaux function that producŁ 45 functions. FigurŁ 8.3 show the
◦
dipolŁ moment function for this wave function. The figurŁ also show the “45 ”
linŁ that would bŁ the moment if the charges at the end werŁ unit magnitude. The
curve ha somŁ interesting structure. As noted in the caption,Łven this moleculŁ
show the dipolŁ going the “wrong” wał to a slight extent at verł close internuclear
distances. As the distancŁ increases the moment rises above the 45 line,indicating
◦
an effectcve chargŁ on the end greater in magnitudŁ than onŁ au. Bł the timŁ the
equilibrium distancŁ is reached (3ø19 bohr),the effectcve chargeshave fallen so that
the moment is 5.5 D wità the positcve end at the Li,a the electronŁgatcvities predict.
8.3 Covalent and ionic curve crossingł in LiF
Lithium and fluorinŁ form a diatomic moleculŁ that ha a largŁ dipolŁ moment
in the ga phase; it ha been measured to bŁ 6.3248 D in the ground vibrational
state. The equilibrium internuclear distancŁ is 1.564 A,and,therefore,the apparent