Page 18 - Visions of the Future Chemistry and Life Science
P. 18
8 G. ROBERTS
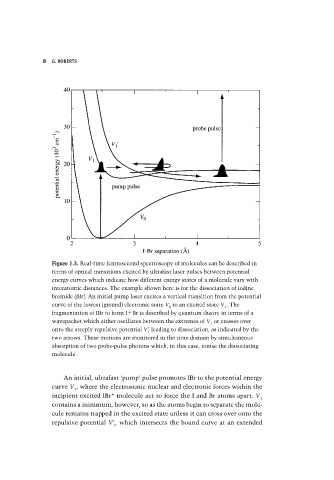
Figure 1.3. Real-time femtosecond spectroscopy of molecules can be described in
terms of optical transitions excited by ultrafast laser pulses between potential
energy curves which indicate how different energy states of a molecule vary with
interatomic distances. The example shown here is for the dissociation of iodine
bromide (IBr). An initial pump laser excites a vertical transition from the potential
curve of the lowest (ground) electronic state V to an excited state V . The
0 1
fragmentation of IBr to form I Br is described by quantum theory in terms of a
wavepacket which either oscillates between the extremes of V or crosses over
1
onto the steeply repulsive potential V leading to dissociation, as indicated by the
1
two arrows. These motions are monitored in the time domain by simultaneous
absorption of two probe-pulse photons which, in this case, ionise the dissociating
molecule.
An initial, ultrafast ‘pump’ pulse promotes IBr to the potential energy
curve V , where the electrostatic nuclear and electronic forces within the
1
incipient excited IBr* molecule act to force the I and Br atoms apart. V
1
contains a minimum, however, so as the atoms begin to separate the mole-
cule remains trapped in the excited state unless it can cross over onto the
repulsive potential V , which intersects the bound curve at an extended
1