Page 23 - Visions of the Future Chemistry and Life Science
P. 23
Laser snapshots of molecular motions 13
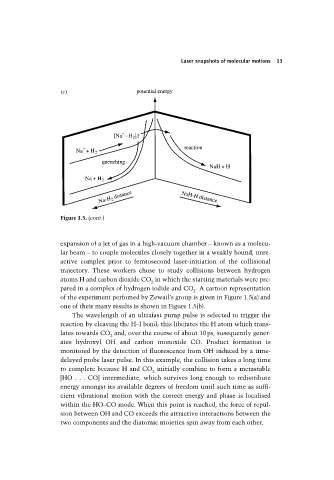
Figure 1.5. (cont.)
expansion of a jet of gas in a high-vacuum chamber – known as a molecu-
lar beam – to couple molecules closely together in a weakly bound, unre-
active complex prior to femtosecond laser-initiation of the collisional
trajectory. These workers chose to study collisions between hydrogen
atoms H and carbon dioxide CO in which the starting materials were pre-
2
pared in a complex of hydrogen iodide and CO . A cartoon representation
2
of the experiment perfomed by Zewail’s group is given in Figure 1.5(a) and
one of their many results is shown in Figure 1.5(b).
The wavelength of an ultrafast pump pulse is selected to trigger the
reaction by cleaving the H–I bond; this liberates the H atom which trans-
lates towards CO and, over the course of about 10ps, susequently gener-
2
ates hydroxyl OH and carbon monoxide CO. Product formation is
monitored by the detection of fluorescence from OH induced by a time-
delayed probe laser pulse. In this example, the collision takes a long time
to complete because H and CO initially combine to form a metastable
2
[HO . . . CO] intermediate, which survives long enough to redistribute
energy amongst its available degrees of freedom until such time as suffi-
cient vibrational motion with the correct energy and phase is localised
within the HO–CO mode. When this point is reached, the force of repul-
sion between OH and CO exceeds the attractive interactions between the
two components and the diatomic moieties spin away from each other.