Page 34 - Visions of the Future Chemistry and Life Science
P. 34
Enzymology takes a quantum leap forward 23
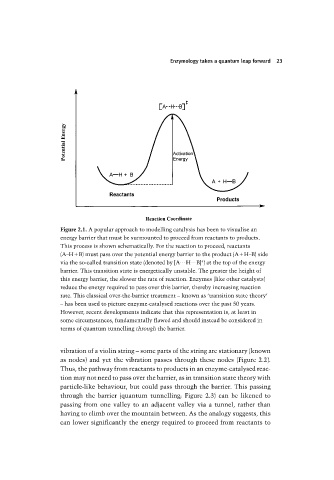
Figure 2.1. A popular approach to modelling catalysis has been to visualise an
energy barrier that must be surmounted to proceed from reactants to products.
This process is shown schematically. For the reaction to proceed, reactants
(A–H B) must pass over the potential energy barrier to the product (A H–B) side
‡
via the so-called transition state (denoted by [A . . . H . . . B] ) at the top of the energy
barrier. This transition state is energetically unstable. The greater the height of
this energy barrier, the slower the rate of reaction. Enzymes (like other catalysts)
reduce the energy required to pass over this barrier, thereby increasing reaction
rate. This classical over-the-barrier treatment – known as ‘transition state theory’
– has been used to picture enzyme-catalysed reactions over the past 50 years.
However, recent developments indicate that this representation is, at least in
some circumstances, fundamentally flawed and should instead be considered in
terms of quantum tunnelling through the barrier.
vibration of a violin string – some parts of the string are stationary (known
as nodes) and yet the vibration passes through these nodes (Figure 2.2).
Thus, the pathway from reactants to products in an enzyme-catalysed reac-
tion may not need to pass over the barrier, as in transition state theory with
particle-like behaviour, but could pass through the barrier. This passing
through the barrier (quantum tunnelling; Figure 2.3) can be likened to
passing from one valley to an adjacent valley via a tunnel, rather than
having to climb over the mountain between. As the analogy suggests, this
can lower significantly the energy required to proceed from reactants to