Page 36 - Visions of the Future Chemistry and Life Science
P. 36
Enzymology takes a quantum leap forward 25
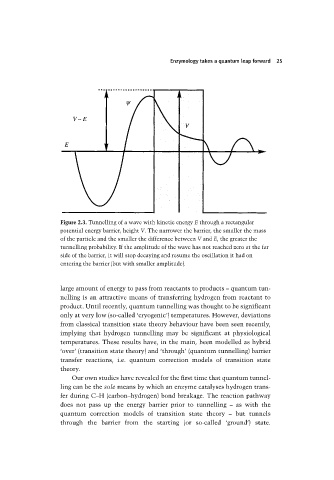
Figure 2.3. Tunnelling of a wave with kinetic energy E through a rectangular
potential energy barrier, height V. The narrower the barrier, the smaller the mass
of the particle and the smaller the difference between V and E, the greater the
tunnelling probability. If the amplitude of the wave has not reached zero at the far
side of the barrier, it will stop decaying and resume the oscillation it had on
entering the barrier (but with smaller amplitude).
large amount of energy to pass from reactants to products – quantum tun-
nelling is an attractive means of transferring hydrogen from reactant to
product. Until recently, quantum tunnelling was thought to be significant
only at very low (so-called ‘cryogenic’) temperatures. However, deviations
from classical transition state theory behaviour have been seen recently,
implying that hydrogen tunnelling may be significant at physiological
temperatures. These results have, in the main, been modelled as hybrid
‘over’ (transition state theory) and ‘through’ (quantum tunnelling) barrier
transfer reactions, i.e. quantum correction models of transition state
theory.
Our own studies have revealed for the first time that quantum tunnel-
ling can be the sole means by which an enzyme catalyses hydrogen trans-
fer during C–H (carbon–hydrogen) bond breakage. The reaction pathway
does not pass up the energy barrier prior to tunnelling – as with the
quantum correction models of transition state theory – but tunnels
through the barrier from the starting (or so-called ‘ground’) state.