Page 109 - Materials Chemistry, Second Edition
P. 109
CAT3525_C04.qxd 1/27/2005 11:12 AM Page 80
80 Waste Management Practices: Municipal, Hazardous, and Industrial
4.5.1 ULTIMATE ANALYSIS OF SOLID WASTE COMPONENTS
The ultimate analysis of a material is defined as its total elemental analysis, i.e., the percentage of
each individual element present. The results of the ultimate analysis are typically used to charac-
terize the chemical composition of the organic fraction of MSW. Such a determination is essential
for assessing the suitability of the waste as a fuel and predicting emissions from combustion. The
data are also used to define the proper mix of MSW materials to achieve suitable nutrient ratios
(e.g., C/N) for biological conversion processes such as composting.
The ultimate analysis involves the determination of the percent values of carbon, hydrogen,
oxygen, nitrogen, sulfur, and ash in a sample. Due to concerns over emissions of chlorinated com-
pounds during combustion, the determination of halogens is often included in an ultimate analysis
(Tchobanoglous et al., 1993; Liu and Liptak, 2000). The percent values of carbon, hydrogen, nitro-
gen, sulfur, and chlorine are measured directly by established procedures. The oxygen value is cal-
culated by subtracting the other components, including ash and moisture, from 100%.
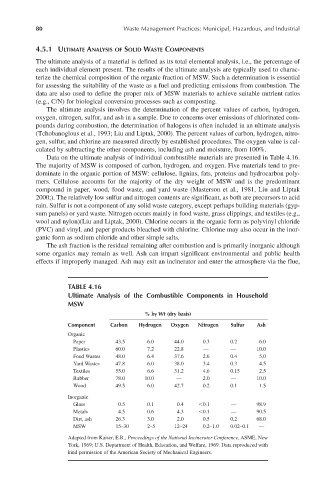
Data on the ultimate analysis of individual combustible materials are presented in Table 4.16.
The majority of MSW is composed of carbon, hydrogen, and oxygen. Five materials tend to pre-
dominate in the organic portion of MSW: cellulose, lignins, fats, proteins and hydrocarbon poly-
mers. Cellulose accounts for the majority of the dry weight of MSW and is the predominant
compound in paper, wood, food waste, and yard waste (Masterson et al., 1981, Liu and Liptak
2000;). The relatively low sulfur and nitrogen contents are significant, as both are precursors to acid
rain. Sulfur is not a component of any solid waste category, except perhaps building materials (gyp-
sum panels) or yard waste. Nitrogen occurs mainly in food waste, grass clippings, and textiles (e.g.,
wool and nylon)(Liu and Liptak, 2000). Chlorine occurs in the organic form as polyvinyl chloride
(PVC) and vinyl, and paper products bleached with chlorine. Chlorine may also occur in the inor-
ganic form as sodium chloride and other simple salts.
The ash fraction is the residual remaining after combustion and is primarily inorganic although
some organics may remain as well. Ash can impart significant environmental and public health
effects if improperly managed. Ash may exit an incinerator and enter the atmosphere via the flue,
TABLE 4.16
Ultimate Analysis of the Combustible Components in Household
MSW
% by Wt (dry basis)
Component Carbon Hydrogen Oxygen Nitrogen Sulfur Ash
Organic
Paper 43.5 6.0 44.0 0.3 0.2 6.0
Plastics 60.0 7.2 22.8 — — 10.0
Food Wastes 48.0 6.4 37.6 2.6 0.4 5.0
Yard Wastes 47.8 6.0 38.0 3.4 0.3 4.5
Textiles 55.0 6.6 31.2 4.6 0.15 2.5
Rubber 78.0 10.0 — 2.0 — 10.0
Wood 49.5 6.0 42.7 0.2 0.1 1.5
Inorganic
Glass 0.5 0.1 0.4 0.1 — 98.9
Metals 4.5 0.6 4.3 0.1 — 90.5
Dirt, ash 26.3 3.0 2.0 0.5 0.2 68.0
MSW 15–30 2–5 12–24 0.2–1.0 0.02–0.1 —
Adapted from Kaiser, E.R., Proceedings of the National Incinerater Conference, ASME, New
York, 1969; U.S. Department of Health, Education, and Welfare, 1969. Data reproduced with
kind permission of the American Society of Mechanical Engineers.