Page 283 - Materials Chemistry, Second Edition
P. 283
CAT3525_C09.qxd 2/8/2005 10:11 AM Page 254
254 Waste Management Practices: Municipal, Hazardous, and Industrial
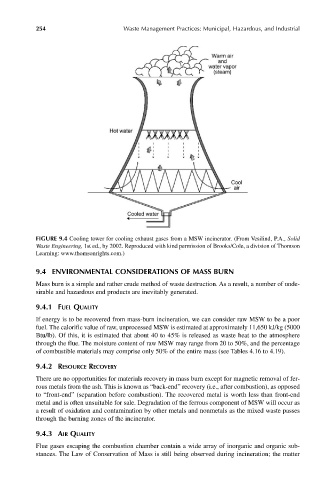
FIGURE 9.4 Cooling tower for cooling exhaust gases from a MSW incinerator. (From Vesilind, P.A., Solid
Waste Engineering, 1st ed., by 2002. Reproduced with kind permission of Brooks/Cole, a division of Thomson
Learning: www.thomsonrights.com.)
9.4 ENVIRONMENTAL CONSIDERATIONS OF MASS BURN
Mass burn is a simple and rather crude method of waste destruction. As a result, a number of unde-
sirable and hazardous end products are inevitably generated.
9.4.1 FUEL QUALITY
If energy is to be recovered from mass-burn incineration, we can consider raw MSW to be a poor
fuel. The calorific value of raw, unprocessed MSW is estimated at approximately 11,650 kJ/kg (5000
Btu/lb). Of this, it is estimated that about 40 to 45% is released as waste heat to the atmosphere
through the flue. The moisture content of raw MSW may range from 20 to 50%, and the percentage
of combustible materials may comprise only 50% of the entire mass (see Tables 4.16 to 4.19).
9.4.2 RESOURCE RECOVERY
There are no opportunities for materials recovery in mass burn except for magnetic removal of fer-
rous metals from the ash. This is known as “back-end” recovery (i.e., after combustion), as opposed
to “front-end” (separation before combustion). The recovered metal is worth less than front-end
metal and is often unsuitable for sale. Degradation of the ferrous component of MSW will occur as
a result of oxidation and contamination by other metals and nonmetals as the mixed waste passes
through the burning zones of the incinerator.
9.4.3 AIR QUALITY
Flue gases escaping the combustion chamber contain a wide array of inorganic and organic sub-
stances. The Law of Conservation of Mass is still being observed during incineration; the matter