Page 290 - Materials Chemistry, Second Edition
P. 290
CAT3525_C09.qxd 2/8/2005 10:11 AM Page 261
Incineration of MSW 261
H
H
H H C
−H C H
HC C H HC H CH
heat H
H H C
H H C
H H
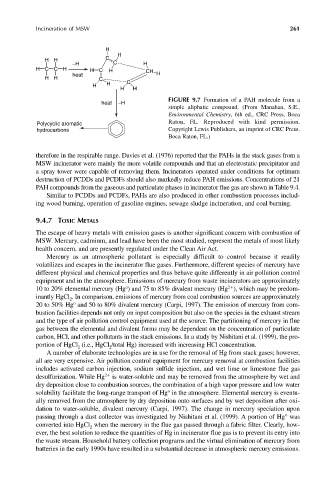
FIGURE 9.7 Formation of a PAH molecule from a
heat −H
simple aliphatic compound. (From Manahan, S.E.,
Environmental Chemistry, 6th ed., CRC Press, Boca
Polycyclic aromatic Raton, FL. Reproduced with kind permission.
hydrocarbons Copyright Lewis Publishers, an imprint of CRC Press.
Boca Raton, FL.)
therefore in the respirable range. Davies et al. (1976) reported that the PAHs in the stack gases from a
MSW incinerator were mainly the more volatile compounds and that an electrostatic precipitator and
a spray tower were capable of removing them. Incinerators operated under conditions for optimum
destruction of PCDDs and PCDFs should also markedly reduce PAH emissions. Concentrations of 21
PAH compounds from the gaseous and particulate phases in incinerator flue gas are shown in Table 9.4.
Similar to PCDDs and PCDFs, PAHs are also produced in other combustion processes includ-
ing wood burning, operation of gasoline engines, sewage sludge incineration, and coal burning.
9.4.7 TOXIC METALS
The escape of heavy metals with emission gases is another significant concern with combustion of
MSW. Mercury, cadmium, and lead have been the most studied, represent the metals of most likely
health concern, and are presently regulated under the Clean Air Act.
Mercury as an atmospheric pollutant is especially difficult to control because it readily
volatilizes and escapes in the incinerator flue gases. Furthermore, different species of mercury have
different physical and chemical properties and thus behave quite differently in air pollution control
equipment and in the atmosphere. Emissions of mercury from waste incinerators are approximately
o
2
10 to 20% elemental mercury (Hg ) and 75 to 85% divalent mercury (Hg ), which may be predom-
inantly HgCl . In comparison, emissions of mercury from coal combustion sources are approximately
2
o
20 to 50% Hg and 50 to 80% divalent mercury (Carpi, 1997). The emission of mercury from com-
bustion facilities depends not only on input composition but also on the species in the exhaust stream
and the type of air pollution control equipment used at the source. The partitioning of mercury in flue
gas between the elemental and divalent forms may be dependent on the concentration of particulate
carbon, HCl, and other pollutants in the stack emissions. In a study by Nishitani et al. (1999), the pro-
portion of HgCl (i.e., HgCl /total Hg) increased with increasing HCl concentration.
2 2
A number of elaborate technologies are in use for the removal of Hg from stack gases; however,
all are very expensive. Air pollution control equipment for mercury removal at combustion facilities
includes activated carbon injection, sodium sulfide injection, and wet lime or limestone flue gas
desulfurization. While Hg 2 is water-soluble and may be removed from the atmosphere by wet and
dry deposition close to combustion sources, the combination of a high vapor pressure and low water
o
solubility facilitate the long-range transport of Hg in the atmosphere. Elemental mercury is eventu-
ally removed from the atmosphere by dry deposition onto surfaces and by wet deposition after oxi-
dation to water-soluble, divalent mercury (Carpi, 1997). The change in mercury speciation upon
passing through a dust collector was investigated by Nishitani et al. (1999). A portion of Hg° was
converted into HgCl when the mercury in the flue gas passed through a fabric filter. Clearly, how-
2
ever, the best solution to reduce the quantities of Hg in incinerator flue gas is to prevent its entry into
the waste stream. Household battery collection programs and the virtual elimination of mercury from
batteries in the early 1990s have resulted in a substantial decrease in atmospheric mercury emissions.