Page 325 - Wastewater Solids Incineration Systems
P. 325
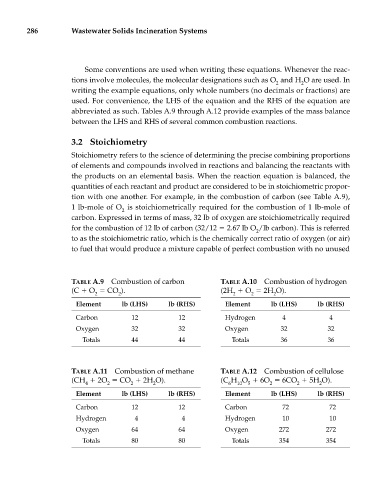
286 Wastewater Solids Incineration Systems
Some conventions are used when writing these equations. Whenever the reac-
tions involve molecules, the molecular designations such as O and H O are used. In
2 2
writing the example equations, only whole numbers (no decimals or fractions) are
used. For convenience, the LHS of the equation and the RHS of the equation are
abbreviated as such. Tables A.9 through A.12 provide examples of the mass balance
between the LHS and RHS of several common combustion reactions.
3.2 Stoichiometry
Stoichiometry refers to the science of determining the precise combining proportions
of elements and compounds involved in reactions and balancing the reactants with
the products on an elemental basis. When the reaction equation is balanced, the
quantities of each reactant and product are considered to be in stoichiometric propor-
tion with one another. For example, in the combustion of carbon (see Table A.9),
1 lb-mole of O is stoichiometrically required for the combustion of 1 lb-mole of
2
carbon. Expressed in terms of mass, 32 lb of oxygen are stoichiometrically required
for the combustion of 12 lb of carbon (32/12 2.67 lb O /lb carbon). This is referred
2
to as the stoichiometric ratio, which is the chemically correct ratio of oxygen (or air)
to fuel that would produce a mixture capable of perfect combustion with no unused
TABLE A.9 Combustion of carbon TABLE A.10 Combustion of hydrogen
(C O CO ). (2H O 2H O).
2 2 2 2 2
Element lb (LHS) lb (RHS) Element lb (LHS) lb (RHS)
Carbon 12 12 Hydrogen 4 4
Oxygen 32 32 Oxygen 32 32
Totals 44 44 Totals 36 36
TABLE A.11 Combustion of methane TABLE A.12 Combustion of cellulose
(CH 2O CO 2H O). (C H O 6O 6CO 5H O).
4 2 2 2 6 10 5 2 2 2
Element lb (LHS) lb (RHS) Element lb (LHS) lb (RHS)
Carbon 12 12 Carbon 72 72
Hydrogen 4 4 Hydrogen 10 10
Oxygen 64 64 Oxygen 272 272
Totals 80 80 Totals 354 354