Page 320 - Wastewater Solids Incineration Systems
P. 320
Appendix A Combustion Fundamentals 281
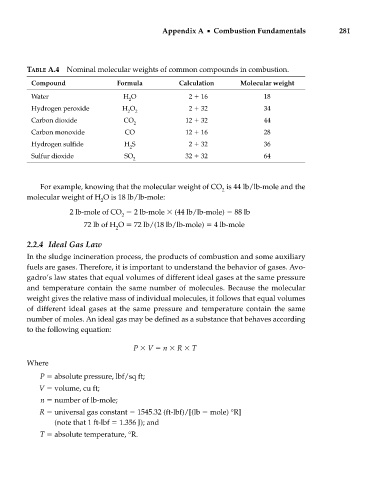
TABLE A.4 Nominal molecular weights of common compounds in combustion.
Compound Formula Calculation Molecular weight
Water H O 2 16 18
2
Hydrogen peroxide H O 2 32 34
2 2
Carbon dioxide CO 12 32 44
2
Carbon monoxide CO 12 16 28
Hydrogen sulfide H S 2 32 36
2
Sulfur dioxide SO 32 32 64
2
For example, knowing that the molecular weight of CO is 44 lb/lb-mole and the
2
molecular weight of H O is 18 lb/lb-mole:
2
2 lb-mole of CO 2 lb-mole (44 lb/lb-mole) 88 lb
2
72 lb of H O 72 lb/(18 lb/lb-mole) 4 lb-mole
2
2.2.4 Ideal Gas Law
In the sludge incineration process, the products of combustion and some auxiliary
fuels are gases. Therefore, it is important to understand the behavior of gases. Avo-
gadro’s law states that equal volumes of different ideal gases at the same pressure
and temperature contain the same number of molecules. Because the molecular
weight gives the relative mass of individual molecules, it follows that equal volumes
of different ideal gases at the same pressure and temperature contain the same
number of moles. An ideal gas may be defined as a substance that behaves according
to the following equation:
P V n R T
Where
P absolute pressure, lbf/sq ft;
V volume, cu ft;
n number of lb-mole;
R universal gas constant 1545.32 (ft-lbf)/[(lb mole) °R]
(note that 1 ft-lbf 1.356 J); and
T absolute temperature, °R.