Page 324 - Wastewater Solids Incineration Systems
P. 324
Appendix A Combustion Fundamentals 285
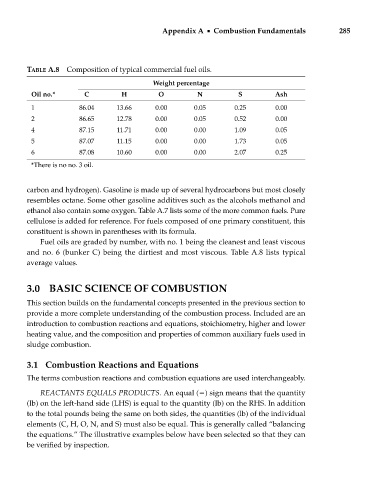
TABLE A.8 Composition of typical commercial fuel oils.
Weight percentage
Oil no.* C H O N S Ash
1 86.04 13.66 0.00 0.05 0.25 0.00
2 86.65 12.78 0.00 0.05 0.52 0.00
4 87.15 11.71 0.00 0.00 1.09 0.05
5 87.07 11.15 0.00 0.00 1.73 0.05
6 87.08 10.60 0.00 0.00 2.07 0.25
*There is no no. 3 oil.
carbon and hydrogen). Gasoline is made up of several hydrocarbons but most closely
resembles octane. Some other gasoline additives such as the alcohols methanol and
ethanol also contain some oxygen. Table A.7 lists some of the more common fuels. Pure
cellulose is added for reference. For fuels composed of one primary constituent, this
constituent is shown in parentheses with its formula.
Fuel oils are graded by number, with no. 1 being the cleanest and least viscous
and no. 6 (bunker C) being the dirtiest and most viscous. Table A.8 lists typical
average values.
3.0 BASIC SCIENCE OF COMBUSTION
This section builds on the fundamental concepts presented in the previous section to
provide a more complete understanding of the combustion process. Included are an
introduction to combustion reactions and equations, stoichiometry, higher and lower
heating value, and the composition and properties of common auxiliary fuels used in
sludge combustion.
3.1 Combustion Reactions and Equations
The terms combustion reactions and combustion equations are used interchangeably.
REACTANTS EQUALS PRODUCTS. An equal ( ) sign means that the quantity
(lb) on the left-hand side (LHS) is equal to the quantity (lb) on the RHS. In addition
to the total pounds being the same on both sides, the quantities (lb) of the individual
elements (C, H, O, N, and S) must also be equal. This is generally called “balancing
the equations.” The illustrative examples below have been selected so that they can
be verified by inspection.