Page 297 - Water and wastewater engineering
P. 297
7-14 WATER AND WASTEWATER ENGINEERING
“First Stage”
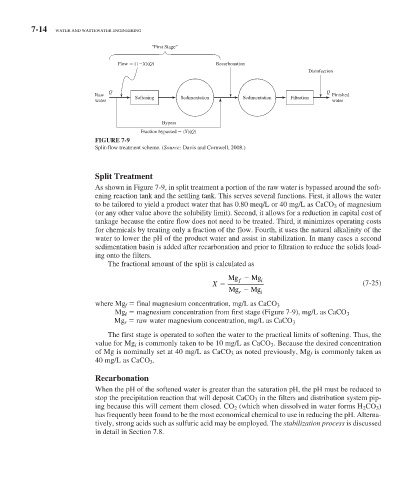
Flow (1 X)(Q) Recarbonation
Disinfection
Q Q
Raw Softening Sedimentation Sedimentation Filtration Finished
water water
Bypass
Fraction bypassed (X)(Q)
FIGURE 7-9
Split-flow treatment scheme. (Source: Davis and Cornwell, 2008.)
Split Treatment
As shown in Figure 7-9 , in split treatment a portion of the raw water is bypassed around the soft-
ening reaction tank and the settling tank. This serves several functions. First, it allows the water
to be tailored to yield a product water that has 0.80 meq/L or 40 mg/L as CaCO 3 of magnesium
(or any other value above the solubility limit). Second, it allows for a reduction in capital cost of
tankage because the entire flow does not need to be treated. Third, it minimizes operating costs
for chemicals by treating only a fraction of the flow. Fourth, it uses the natural alkalinity of the
water to lower the pH of the product water and assist in stabilization. In many cases a second
sedimentation basin is added after recarbonation and prior to filtration to reduce the solids load-
ing onto the filters.
The fractional amount of the split is calculated as
Mg f Mg i
X (7-25)
Mg r Mg i
where Mg f final magnesium concentration, mg/L as CaCO 3
Mg i magnesium concentration from first stage ( Figure 7-9 ), mg/L as CaCO 3
Mg r raw water magnesium concentration, mg/L as CaCO 3
The first stage is operated to soften the water to the practical limits of softening. Thus, the
value for Mg i is commonly taken to be 10 mg/L as CaCO 3 . Because the desired concentration
of Mg is nominally set at 40 mg/L as CaCO 3 as noted previously, Mg f is commonly taken as
40 mg/L as CaCO 3 .
Recarbonation
When the pH of the softened water is greater than the saturation pH, the pH must be reduced to
stop the precipitation reaction that will deposit CaCO 3 in the filters and distribution system pip-
ing because this will cement them closed. CO 2 (which when dissolved in water forms H 2 CO 3 )
has frequently been found to be the most economical chemical to use in reducing the pH. Alterna-
tively, strong acids such as sulfuric acid may be employed. The stabilization process is discussed
in detail in Section 7.8.