Page 168 - Well Logging and Formation Evaluation
P. 168
158 Well Logging and Formation Evaluation
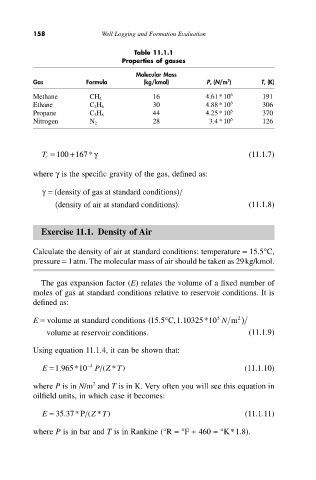
Table 11.1.1
Properties of gasses
Molecular Mass
Gas Formula (kg/kmol) P c (N/m ) 2 T c (K)
Methane CH 4 16 4.61*10 6 191
Ethane C 2H 6 30 4.88*10 6 306
Propane C 3H 8 44 4.25*10 6 370
Nitrogen N 2 28 3.4*10 6 126
T c = 100 +167* g (11.1.7)
where g is the specific gravity of the gas, defined as:
g= (density of gas at standard conditions )
(density of air at standard conditions ). (11.1.8)
Exercise 11.1. Density of Air
Calculate the density of air at standard conditions: temperature = 15.5°C,
pressure = 1atm. The molecular mass of air should be taken as 29kg/kmol.
The gas expansion factor (E) relates the volume of a fixed number of
moles of gas at standard conditions relative to reservoir conditions. It is
defined as:
E = volume at standard conditions (15.5 ∞C,1.10325*10 5 N m 2 )
volume at reservoir conditions. (11.1.9)
Using equation 11.1.4, it can be shown that:
*
( *
.
E = 1 965 10 -4 P Z T) (11.1.10)
2
where P is in N/m and T is in K. Very often you will see this equation in
oilfield units, in which case it becomes:
Z T)
E = 35 37* P ( * (11.1.11)
.
where P is in bar and T is in Rankine (°R = °F + 460 = °K*1.8).