Page 160 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 160
QC: IML/FFX
P1: IML/FFX
AT029-Manual
AT029-03
June 22, 2007
140 CHARACTERIZATION AND PROPERTIES OF PETROLEUM FRACTIONS
Research Octane Number P2: IML/FFX AT029-Manual-v7.cls T1: IML 14:23 Research Octane Number
n
n n
n
Boiling Point, °C
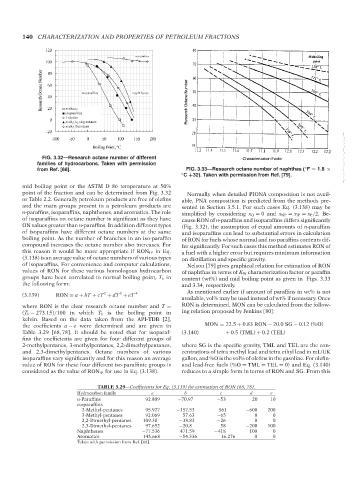
FIG. 3.32—Research octane number of different Characterization Factor --`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
families of hydrocarbons. Taken with permission
◦
from Ref. [68]. FIG. 3.33—Research octane number of naphthas ( F = 1.8 ×
◦ C +32). Taken with permission from Ref. [79].
mid boiling point or the ASTM D 86 temperature at 50%
point of the fraction and can be determined from Fig. 3.32 Normally when detailed PIONA composition is not avail-
or Table 2.2. Generally petroleum products are free of olefins able, PNA composition is predicted from the methods pre-
and the main groups present in a petroleum products are sented in Section 3.5.1. For such cases Eq. (3.138) may be
n-paraffins, isoparaffins, naphthenes, and aromatics. The role simplified by considering x O = 0 and x NP = x IP = x P /2. Be-
of isoparaffins on octane number is significant as they have cause RON of n-paraffins and isoparaffins differs significantly
ON values greater than n-paraffins. In addition different types (Fig. 3.32), the assumption of equal amounts of n-paraffins
of isoparaffins have different octane numbers at the same and isoparaffins can lead to substantial errors in calculation
boiling point. As the number of branches in an iso-paraffin of RON for fuels whose normal and iso paraffins contents dif-
compound increases the octane number also increases. For fer significantly. For such cases this method estimates RON of
this reason it would be more appropriate if RON IP in Eq. a fuel with a higher error but requires minimum information
(3.138) is an average value of octane numbers of various types on distillation and specific gravity.
of isoparaffins. For convenience and computer calculations, Nelson [79] gives graphical relation for estimation of RON
values of RON for these various homologous hydrocarbon of naphthas in terms of K W characterization factor or paraffin
groups have been correlated to normal boiling point, T b in content (wt%) and mid boiling point as given in Figs. 3.33
the following form: and 3.34, respectively.
As mentioned earlier if amount of paraffins in wt% is not
4
2
3
(3.139) RON = a + bT + cT + dT + eT
available, vol% may be used instead of wt% if necessary. Once
RON is determined, MON can be calculated from the follow-
where RON is the clear research octane number and T =
(T b − 273.15)/100 in which T b is the boiling point in ing relation proposed by Jenkins [80]:
kelvin. Based on the data taken from the API-TDB [2],
the coefficients a − e were determined and are given in MON = 22.5 + 0.83 RON − 20.0SG − 0.12 (%O)
Table 3.29 [68, 78]. It should be noted that for isoparaf- (3.140) + 0.5 (TML) + 0.2 (TEL)
fins the coefficients are given for four different groups of
2-methylpentanes, 3-methylpentanes, 2,2-dimethylpentanes, where SG is the specific gravity, TML and TEL are the con-
and 2,3-dimethylpentanes. Octane numbers of various centrations of tetra methyl lead and tetra ethyl lead in mL/UK
isoparaffins vary significantly and for this reason an average gallon, and %O is the vol% of olefins in the gasoline. For olefin-
value of RON for these four different iso-paraffinic groups is and lead-free fuels (%O = TML = TEL = 0) and Eq. (3.140)
considered as the value of RON IP for use in Eq. (3.138). reduces to a simple form in terms of RON and SG. From this
TABLE 3.29—Coefficients for Eq. (3.139) for estimation of RON [68, 78].
Hydrocarbon family a b c d e
n-Paraffins 92.809 −70.97 −53 20 10
isoparaffins
2-Methyl-pentanes 95.927 −157.53 561 −600 200
3-Methyl-pentanes 92.069 57.63 −65 0 0
2,2-Dimethyl-pentanes 109.38 −38.83 −26 0 0
2,3-Dimethyl-pentanes 97.652 −20.8 58 −200 100
Naphthenes −77.536 471.59 −418 100 0
Aromatics 145.668 −54.336 16.276 0 0
Taken with permission from Ref. [68].
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT