Page 161 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 161
P2: IML/FFX
QC: IML/FFX
T1: IML
P1: IML/FFX
June 22, 2007
14:23
AT029-03
AT029-Manual-v7.cls
AT029-Manual
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
3. CHARACTERIZATION OF PETROLEUM FRACTIONS 141
b. To predict PNA, we calculate M from Eq. (2.50) as M =
79.54. Since M < 200 and viscosity is not available we use
Eqs. (3.77) and (3.78) and (3.72) to predict the composi-
tion. From Eqs. (2.126) and (2.127), n 20 = 1.3642 and from
Eq. (3.50), m =−8.8159. The predicted composition for
%P, %N, and %A is 95.4%, 7.4%, and −2.8%, respectively.
Research Octane Number the normalized composition is x P = 0.928, x N = 0.072, and
Since predicted %A is negative, it is set equal to zero and
x A = 0.0. To use Eq. (3.139) we split the paraffin content
equally between n-paraffins and isoparaffins as x NP = x IP =
0.928/2 = 0.464. In this case RON = 72.36. The error on
calculation of RON is 2.76.
c. To use Fig. 3.33 we need T b = 42.8 C = 109 F and K W ,
◦
◦
which from Eq. (2.13) is calculated as K W = 12.75. Since
the K W is outside the range of values in Fig. 3.33, accurate
reading is not possible, but from value of the boiling point
it is obvious that the RON from extrapolation of the curves
is above 70.
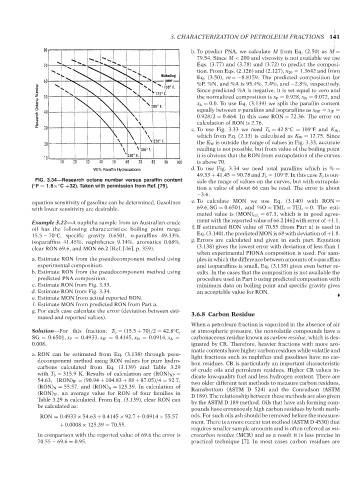
Wt% Paraffin Hydrocarbons d. To use Fig. 3.34 we need total paraffins which is % =
49.33 + 41.45 = 90.78 and T b = 109 F. In this case T b is out-
◦
FIG. 3.34—Research octane number versus paraffin content side the range of values on the curves, but with extrapola-
( F = 1.8× C +32). Taken with permission from Ref. [79].
◦
◦
tion a value of about 66 can be read. The error is about
−3.6.
equation sensitivity of gasoline can be determined. Gasolines e. To calculate MON we use Eq. (3.140) with RON =
with lower sensitivity are desirable. 69.6, SG = 0.6501, and %O = TML = TEL = 0. The esti-
mated value is (MON) est. = 67.3, which is in good agree-
Example 3.22—A naphtha sample from an Australian crude ment with the reported value of 66.2 [46] with error of +1.1.
oil has the following characteristics: boiling point range f. If estimated RON value of 70.55 (from Part a) is used in
15.5 − 70 C, specific gravity 0.6501, n-paraffins 49.33%, Eq. (3.140), the predicted MON is 68 with deviation of +1.8.
◦
isoparaffins 41.45%, naphthenes 9.14%, aromatics 0.08%, g. Errors are calculated and given in each part. Equation
clear RON 69.6, and MON 66.2 [Ref. [46], p. 359). (3.138) gives the lowest error with deviation of less than 1
when experimental PIONA composition is used. For sam-
a. Estimate RON from the pseudocomponent method using ples in which the difference between amounts of n-paraffins
experimental composition. and isoparaffins is small, Eq. (3.138) gives even better re-
b. Estimate RON from the pseudocomponent method using sults. In the cases that the composition is not available the
predicted PNA composition. procedure used in Part b using predicted composition with
c. Estimate RON from Fig. 3.33. minimum data on boiling point and specific gravity gives
d. Estimate RON from Fig. 3.34. an acceptable value for RON.
e. Estimate MON from actual reported RON.
f. Estimate MON from predicted RON from Part a.
g. For each case calculate the error (deviation between esti- 3.6.8 Carbon Residue
mated and reported values).
When a petroleum fraction is vaporized in the absence of air
Solution—For this fraction: T b = (15.5 + 70)/2 = 42.8 C, at atmospheric pressure, the nonvolatile compounds have a
◦
SG = 0.6501, x P = 0.4933, x IP = 0.4145, x N = 0.0914, x A = carbonaceous residue known as carbon residue, which is des-
0.008. ignated by CR. Therefore, heavier fractions with more aro-
matic contents have higher carbon residues while volatile and
a. RON can be estimated from Eq. (3.138) through pseu- light fractions such as naphthas and gasolines have no car-
docomponent method using RON values for pure hydro- bon residues. CR is particularly an important characteristic
carbons calculated from Eq. (3.139) and Table 3.29
of crude oils and petroleum residues. Higher CR values in-
with T b = 315.9 K. Results of calculation are (RON) NP = dicate low-quality fuel and less hydrogen content. There are
54.63, (RON) IP = (90.94 + 104.83 + 88 + 87.05)/4 = 92.7, two older different test methods to measure carbon residues,
(RON) N = 55.57, and (RON) A = 125.39. In calculation of Ramsbottom (ASTM D 524) and the Conradson (ASTM
(RON) IP , an average value for RON of four families in D 189). The relationship between these methods are also given
Table 3.29 is calculated. From Eq. (3.139), clear RON can by the ASTM D 189 method. Oils that have ash forming com-
be calculated as:
pounds have erroneously high carbon residues by both meth-
RON = 0.4933 × 54.63 + 0.4145 × 92.7 + 0.0914 × 55.57 ods. For such oils ash should be removed before the measure-
ment. There is a more recent test method (ASTM D 4530) that
+ 0.0008 × 125.39 = 70.55.
requires smaller sample amounts and is often referred as mi-
In comparison with the reported value of 69.6 the error is crocarbon residue (MCR) and as a result it is less precise in
70.55 − 69.6 = 0.95. practical technique [7]. In most cases carbon residues are
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT