Page 236 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 236
P2: IML/FFX
QC: IML/FFX
P1: IML/FFX
AT029-Manual
17:42
August 16, 2007
AT029-05
216 CHARACTERIZATION AND PROPERTIES OF PETROLEUM FRACTIONS
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
Compressibility Factor, Z AT029-Manual-v7.cls T1: IML Reduced Pressure, Pr Compressibility Factor, Z
Reduced Pressure, Pr
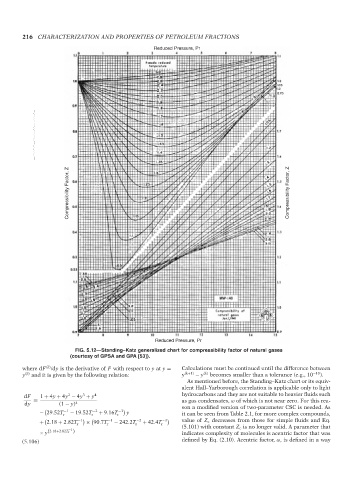
FIG. 5.12—Standing–Katz generalized chart for compressibility factor of natural gases
(courtesy of GPSA and GPA [53]).
(k)
where dF /dy is the derivative of F with respect to y at y = Calculations must be continued until the difference between
y (k) and it is given by the following relation: y (k+1) − y (k) becomes smaller than a tolerance (e.g., 10 −10 ).
As mentioned before, the Standing–Katz chart or its equiv-
alent Hall–Yarborough correlation is applicable only to light
2
3
dF 1 + 4y + 4y − 4y + y 4 hydrocarbons and they are not suitable to heavier fluids such
= as gas condensates, ω of which is not near zero. For this rea-
dy (1 − y) 4
son a modified version of two-parameter CSC is needed. As
− 29.52T r −1 − 19.52T r −2 + 9.16T r −3 y it can be seen from Table 2.1, for more complex compounds,
+ 2.18 + 2.82T r −1 × 90.7T r −1 − 242.2T r −2 + 42.4T r −3 value of Z c decreases from those for simple fluids and Eq.
(5.101) with constant Z c is no longer valid. A parameter that
−1
× y ( 2.18+2.82T r ) indicates complexity of molecules is acentric factor that was
(5.106) defined by Eq. (2.10). Acentric factor, ω, is defined in a way
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT