Page 310 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 310
P2: KVU/KXT
QC: —/—
T1: IML
P1: KVU/KXT
June 22, 2007
20:46
AT029-Manual
AT029-06
AT029-Manual-v7.cls
290 CHARACTERIZATION AND PROPERTIES OF PETROLEUM FRACTIONS
where parameters a 1 and a 2 are first and second derivatives of
of sound in gases and liquids.
EOS parameter a with respect to temperature and for both RK TABLE 6.18—RK and PR EOS parameters (Eq. 6.242) from velocity --`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
and PR equations are given in Table 6.1. In terms of parame- Compound No. of RK EOS a PR EOS a
ters a and b, velocity of sound equation for both RK and SRK (gas) points α s β s α s β s
77
are the same. Their difference lies in calculation of parameter Methane 119 1.025 1.111 0.936 1.093
1.123
0.956
1.043
0.993
Ethane
a through Eq. (5.41), where for RK EOS, α = 1. Now we de- Propane 63 0.992 1.026 1.013 1.031
fine EOS parameters determined from cVT data in terms of Isobutane 80 0.993 1.019 0.983 0.983
original EOS parameters (a EOS and b EOS as given in Table 5.1) n-Butane 86 0.987 1.015 0.941 0.912
in the following forms: n-Pentane (liquid) 1.04 0.9
n-Decane (liquid) 1.06 0.99
Taken with permission from Ref. [8].
a
a s = α s a EOS These parameters must be used for gaseous phase with Eq. (5.40) and param-
(6.242) eters a EOS and b EOS from Tables 5.1.
b s = β s b EOS
Parameters α s and β s can be determined for each compound
or mixtures of constant composition from velocity of sound mixtures it would be appropriate to determine a s and b s from
data. Parameters a EOS and b EOS can be calculated from their cVT data and directly use them in the corresponding EOS
definition and use of critical constants. In fact values of the without calculation of a EOS and b EOS through critical proper-
critical constants used in the calculations do not affect the ties. Therefore, for both RK and SRK we get same values of
outcome of results but they affect calculated values of α s and a s and b s since the original form of EOS is the same. For a
β s . For this reason α s and β s must be used with the same a EOS number of light gases, parameters α s and β s have been deter-
and b EOS that were used originally to determine these param- mined from sonic velocity data for both RK and PR EOSs and
eters. As an alternative approach and especially for petroleum they are given in Table 6.18. Once these parameters are used
TABLE 6.19—Prediction of thermodynamic properties of light gases from RK and PR
equations with use of velocity of sound and original parameters. a
%AAD for RK EOS %AAD for PR EOS
No. of data Sonic Sonic
Gas system points Property velocity Original velocity Original
Pure gas 425 C 0.82 0.58 0.62 0.82
compounds 425 Z 0.76 0.92 0.5 0.77
C 1 ,C 2 ,C 3 , 341 C P 1.9 1.8 1.3 1.2
iC 4 , nC 4 341 H 0.66 0.53 0.42 0.48
Gas mixture 61 C 9.2 0.84 1.47 0.89
69 Mol% C 1 , 66 Z 4.1 2.0 4.0 1.9
31 Mol% C 2 66 C V 8.2 2.85 6.5 7.0
Taken with permission from Ref. [8].
a For the velocity of sound parameters, values of α s and β s from Table 6.18 have been used. For the original
parameters these corrections factors are taken as unity.
100
Original Constant
Velocity of Sound
90 Experimental Data
Cp (J/mol.K) 80
70
60
350 400 450
Temperature (K)
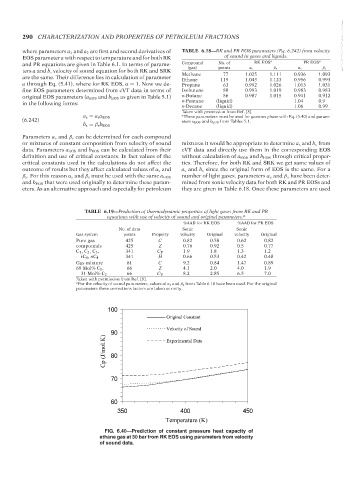
FIG. 6.40—Prediction of constant pressure heat capacity of
ethane gas at 30 bar from RK EOS using parameters from velocity
of sound data.
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT