Page 408 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 408
P2: IML/FFX
QC: IML/FFX
T1: IML
P1: IML/FFX
AT029-Manual
14:25
AT029-Manual-v7.cls
June 22, 2007
AT029-09
388 CHARACTERIZATION AND PROPERTIES OF PETROLEUM FRACTIONS
TABLE 9.11—Constants in Eq. 9.42 for estimation of asphaltene and resin contents of crude oils.
Constants in Eq. (9.42) Absolute Dev.%
Composition type (wt%) Range of API gravity of oil Range of wt% a b c No. of oils Avg Max
Asphaltene in oil 5.9–43.4 0.1–20 −731 674 31 122 1.4 −5.2
Resin in oil 5.5–43.4 5.6–40 −2511.5 2467 −76 41 1.9 −4.9
were mostly obtained from Speight [15] and the Oil and Gas precipitation appropriate thermodynamic models as intro-
Journal Data Book [54]. For prediction of amount of asphal- duced in this section should be used.
tene precipitation when it is diluted by an n-alkane solvent,
the following correlation was developed based on very limited 9.5 VAPOR–SOLID EQUILIBRIUM—
data [53]:
HYDRATE FORMATION
Asphaltene predicted, wt% = a + b(R i) + c (SG)
In this section, another application of phase equilibrium in
(9.43) + d (R S) + e (M S)
the petroleum industry is demonstrated for prediction of hy-
drate formation from vapor–solid equilibrium (VSE) calcula-
where coefficients a–e are determined from experimental
data. Parameters R i and SG are the same as in Eq. (9.42) tions. Hydrates are molecules of gas (C 1 ,C 2 ,C 3 , iC 4 , nC 4 ,
and should be calculated in the same way. M S is the solvent N 2 ,CO 2 ,orH 2 S) dissolved in solid crystals of water. Gas
molecular weight (n-alkane) and R S is the solvent-to-oil ratio molecules, in fact, occupy the void spaces in water crystal
3
in cm /g. This correlation was developed based on the data lattice and the form resembles wet snow. In the oil fields
available for three different Kuwaiti oils and 45 data points, hydrates look like grayish snow cone [1]. Gas hydrates are
and for this limited database the coefficients were deter- solid, semistable compounds that can cause plugging in natu-
mined as a =−2332, b = 2325, c =−112.6, d = 0.0737, and ral gas transmission pipelines, gas handling equipments, noz-
e =−0.0265. With these coefficients the above equation pre- zles, and gas separation units. Gas hydrates may be formed at
◦
dicts asphaltene precipitation of Kuwait oils with AD of 0.5%. temperatures below 35 C when a gas is in contact with water.
The correlation is not appropriate for other crude oils and However, at high pressures (>1000 bar), hydrate formation
◦
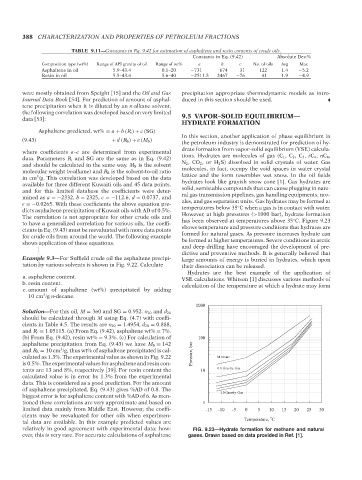
to have a generalized correlation for various oils, the coeffi- has been observed at temperatures above 35 C. Figure 9.23
cients in Eq. (9.43) must be reevaluated with more data points shows temperature and pressure conditions that hydrates are
for crude oils from around the world. The following example formed for natural gases. As pressure increases hydrate can
shows application of these equations. be formed at higher temperatures. Severe conditions in arctic
and deep drilling have encouraged the development of pre-
dictive and preventive methods. It is generally believed that
Example 9.3—For Suffield crude oil the asphaltene precipi- large amounts of energy is buried in hydrates, which upon
tation by various solvents is shown in Fig. 9.22. Calculate their dissociation can be released.
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
Hydrates are the best example of the application of
a. asphaltene content. VSE calculations. Whitson [1] discusses various methods of
b. resin content. calculation of the temperature at which a hydrate may form
c. amount of asphaltene (wt%) precipitated by adding
3
10 cm /g n-decane.
1000
Solution—For this oil, M = 360 and SG = 0.952. n 20 and d 20
should be calculated through M using Eq. (4.7) with coeffi-
cients in Table 4.5. The results are n 20 = 1.4954, d 20 = 0.888,
and R i = 1.05115. (a) From Eq. (9.42), asphaltene wt% = 7%.
(b) From Eq. (9.42), resin wt% = 9.3%. (c) For calculation of 100
asphaltene precipitation from Eq. (9.43) we have M S = 142
3
and R S = 10 cm /g, thus wt% of asphaltene precipitated is cal- Pressure, bar
culated as 1.3%. The experimental value as shown in Fig. 9.22 Methane
is 0.5%. The experimental values for asphaltene and resin con-
tents are 13 and 8%, respectively [39]. For resin content the 10 0.6 Gravity Gas
calculated value is in error by 1.3% from the experimental
data. This is considered as a good prediction. For the amount
of asphaltene precipitated, Eq. (9.43) gives %AD of 0.8. The 1.0 Gravity Gas
biggest error is for asphaltene content with %AD of 6. As men-
tioned these correlations are very approximate and based on 1
limited data mainly from Middle East. However, the coeffi- -15 -10 -5 0 5 10 15 20 25 30
cients may be reevaluated for other oils when experimen- o
tal data are available. In this example predicted values are Temperature, C
relatively in good agreement with experimental data; how- FIG. 9.23—Hydrate formation for methane and natural
ever, this is very rare. For accurate calculations of asphaltene gases. Drawn based on data provided in Ref. [1].
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT