Page 423 -
P. 423
13.20 CHAPTER THIRTEEN
180
160
J
J
140
f
LJ
c~
o_
120
CO jf
t~
J
...J
100
E
80 J
E
.o_ 60
o~
40
20
0
0 5 10 15 20 25 30 35 40
Temperature, °C
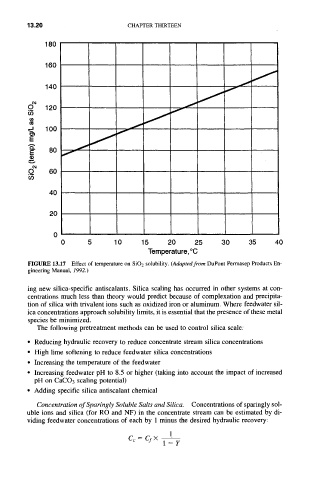
FIGURE 13.17 Effect of temperature on SiO2 solubility. (Adapted from DuPont Permasep Products En-
gineering Manual, 1992.)
ing new silica-specific antiscalants. Silica scaling has occurred in other systems at con-
centrations much less than theory would predict because of complexation and precipita-
tion of silica with trivalent ions such as oxidized iron or aluminum. Where feedwater sil-
ica concentrations approach solubility limits, it is essential that the presence of these metal
species be minimized.
The following pretreatment methods can be used to control silica scale:
• Reducing hydraulic recovery to reduce concentrate stream silica concentrations
• High lime softening to reduce feedwater silica concentrations
• Increasing the temperature of the feedwater
• Increasing feedwater pH to 8.5 or higher (taking into account the impact of increased
pH on CaCO3 scaling potential)
• Adding specific silica antiscalant chemical
Concentration of Sparingly Soluble Salts and Silica. Concentrations of sparingly sol-
uble ions and silica (for RO and NF) in the concentrate stream can be estimated by di-
viding feedwater concentrations of each by 1 minus the desired hydraulic recovery:
1
Cc=Cf× 1- Y