Page 420 -
P. 420
MEMBRANE PROCESSES 13,17
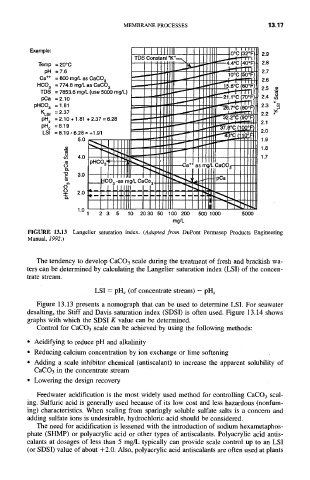
Example: Ooc (32OF
2.9
2.8
Temp =20°C ~ -- . ( F)
pH = 7.6 ~ 2.7
Ca +* = 800 mg/L as CaCO. ~ 10°C (50°1 =)
2.6
HCO 3 =774.8mg/LasCaCO 3 ~ 15.6oC(60oF""r-7~rr~
2.5 m
TDS = 7853.6 mg/L (use 50()0 nag/L) ~ ~ 1 1
-21.1°(:: (70°F) 2.4 ~
pCa = 2 10 ~
1"8 . I I ILIJ.tg,.---"l~ I IIIIIL .....b--+"t'~l/
pHCO, --2:3; ! ooF) 2.3 ~,
v
2.2 =
KLSIpH s = :).10 + 1.81 + 2.37 = 6.28 2.2°(:: (90*[=
2.1
pH c = 8.19 ~ I ~ o c ~ i
2.0
LSI = 8.19 - 6.28 = +1,91 3oc (110o
1.9
1.8
1.7
3.0 ~ nn~
I,__~CO 3 -as mg/L CaCo 3 P'~=
2.0
10
1 2 3 5 10 2030 50 100 200 500 1000 5000
mg/L
FIGURE 13.13 Langelier saturation index. (Adapted from DuPont Permasep Products Engineering
Manual, 1992.)
The tendency to develop CaCO3 scale during the treatment of fresh and brackish wa-
ters can be determined by calculating the Langelier saturation index (LSI) of the concen-
trate stream.
LSI = pHc (of concentrate stream) - pHs
Figure 13.13 presents a nomograph that can be used to determine LSI. For seawater
desalting, the Stiff and Davis saturation index (SDSI) is often used. Figure 13.14 shows
graphs with which the SDSI K value can be determined.
Control for CaCO3 scale can be achieved by using the following methods:
• Acidifying to reduce pH and alkalinity
• Reducing calcium concentration by ion exchange or lime softening
• Adding a scale inhibitor chemical (anfiscalant) to increase the apparent solubility of
CaCO3 in the concentrate stream
• Lowering the design recovery
Feedwater acidification is the most widely used method for controlling CaCO3 scal-
ing. Sulfuric acid is generally used because of its low cost and less hazardous (nonfum-
ing) characteristics. When scaling from sparingly soluble sulfate salts is a concern and
adding sulfate ions is undesirable, hydrochloric acid should be considered.
The need for acidification is lessened with the introduction of sodium hexametaphos-
phate (SHMP) or polyacrylic acid or other types of antiscalants. Polyacrylic acid antis-
calants at dosages of less than 5 mg/L typically can provide scale control up to an LSI
(or SDSI) value of about +2.0. Also, polyacrylic acid antiscalants are often used at plants