Page 425 -
P. 425
CHAPTER THIRTEEN
13.22
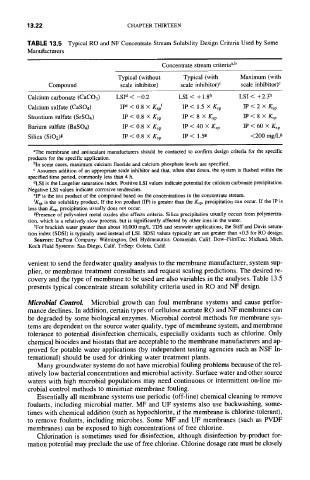
TABLE 13.5 Typical RO and NF Concentrate Stream Solubility Design Criteria Used by Some
Manufacturers
Concentrate stream criteria a'b
Typical (without Typical (with Maximum (with
scale inhibitor) scale inhibitor) c scale inhibitor) c
Compound
Calcium carbonate (CaCO3) LSP < -0.2 LSI < + 1.8 h LSI < +2.3 h
Calcium sulfate (CaSO4) IP e < 0.8 X Ksp f IP < 1.5 X Ksp IP < 2 x Ksp
Strontium sulfate (SrSO4) IP < 0.8 X Ksp IP < 8 X Ksp IP < 8 x Ksp
Barium sulfate (BaSO4) IP < 0.8 x Ksp IP < 40 X Ksp IP < 60 x Ksp
Silica (SiO2) g IP < 0.8 x Ksp IP < 1.5g <200 mg/Lg
aThe membrane and antiscalant manufacturers should be contacted to confirm design criteria for the specific
products for the specific application.
bin some cases, maximum calcium fluoride and calcium phosphate levels are specified.
c Assumes addition of an appropriate scale inhibitor and that, when shut down, the system is flushed within the
specified time period, commonly less than 4 h.
dLSI is the Langelier saturation index. Positive LSI values indicate potential for calcium carbonate precipitation.
Negative LSI values indicate corrosive tendencies.
eIP is the ion product of the compound based on the concentrations in the concentrate stream.
fKsp is the solubility product. If the ion product (IP) is greater than the Ksp, precipitation can occur. If the IP is
less than Ksp, precipitation usually does not occur.
gPresence of polyvalent metal oxides also affects criteria. Silica precipitation usually occurs from polymeriza-
tion, which is a relatively slow process, but is significantly affected by other ions in the water.
hFor brackish water greater than about 10,000 mg/L TDS and seawater applications, the Stiff and Davis satura-
tion index (SDSI) is typically used instead of LSI. SDSI values typically are not greater than +0.5 for RO design.
Sources: DuPont Company: Wilmington, Del. Hydranautics: Oceanside, Calif. Dow-FilmTec: Midland, Mich.
Koch Fluid Systems: San Diego, Calif. TriSep: Goleta, Calif.
venient to send the feedwater quality analysis to the membrane manufacturer, system sup-
plier, or membrane treatment consultants and request scaling predictions. The desired re-
covery and the type of membrane to be used are also variables in the analyses. Table 13.5
presents typical concentrate stream solubility criteria used in RO and NF design.
Microbial Control. Microbial growth can foul membrane systems and cause perfor-
mance declines. In addition, certain types of cellulose acetate RO and NF membranes can
be degraded by some biological enzymes. Microbial control methods for membrane sys-
tems are dependent on the source water quality, type of membrane system, and membrane
tolerance to potential disinfection chemicals, especially oxidants such as chlorine. Only
chemical biocides and biostats that are acceptable to the membrane manufacturers and ap-
proved for potable water applications (by independent testing agencies such as NSF In-
ternational) should be used for drinking water treatment plants.
Many groundwater systems do not have microbial fouling problems because of the rel-
atively low bacterial concentrations and microbial activity. Surface water and other source
waters with high microbial populations may need continuous or intermittent on-line mi-
crobial control methods to minimize membrane fouling.
Essentially all membrane systems use periodic (off-line) chemical cleaning to remove
foulants, including microbial matter. MF and UF systems also use backwashing, some-
times with chemical addition (such as hypochlorite, if the membrane is chlorine-tolerant),
to remove foulants, including microbes. Some MF and UF membranes (such as PVDF
membranes) can be exposed to high concentrations of free chlorine.
Chlorination is sometimes used for disinfection, although disinfection by-product for-
mation potential may preclude the use of free chlorine. Chlorine dosage rate must be closely