Page 231 - A Comprehensive Guide to Solar Energy Systems
P. 231
234 A COMPREHENSIVE GUIdE TO SOLAR ENERGy SySTEMS
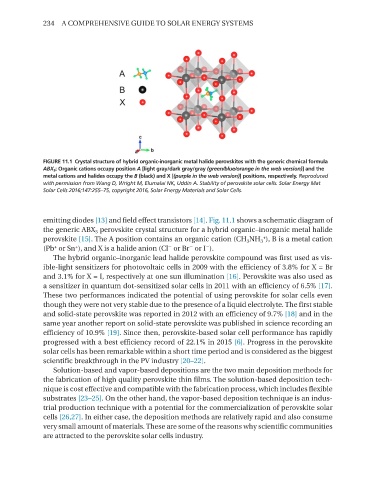
FIGURE 11.1 Crystal structure of hybrid organic-inorganic metal halide perovskites with the generic chemical formula
ABX 3 ; Organic cations occupy position A [light gray/dark gray/gray (green/blue/orange in the web version)] and the
metal cations and halides occupy the B (black) and X [(purple in the web version)] positions, respectively. Reproduced
with permission from Wang D, Wright M, Elumalai NK, Uddin A. Stability of perovskite solar cells. Solar Energy Mat
Solar Cells 2016;147:255–75, copyright 2016, Solar Energy Materials and Solar Cells.
emitting diodes [13] and field effect transistors [14]. Fig. 11.1 shows a schematic diagram of
the generic ABX 3 perovskite crystal structure for a hybrid organic–inorganic metal halide
+
perovskite [15]. The A position contains an organic cation (CH 3 NH 3 ), B is a metal cation
+
+
(Pb or Sn ), and X is a halide anion (Cl or Br or I ).
−
−
−
The hybrid organic–inorganic lead halide perovskite compound was first used as vis-
ible-light sensitizers for photovoltaic cells in 2009 with the efficiency of 3.8% for X = Br
and 3.1% for X = I, respectively at one sun illumination [16]. Perovskite was also used as
a sensitizer in quantum dot-sensitized solar cells in 2011 with an efficiency of 6.5% [17].
These two performances indicated the potential of using perovskite for solar cells even
though they were not very stable due to the presence of a liquid electrolyte. The first stable
and solid-state perovskite was reported in 2012 with an efficiency of 9.7% [18] and in the
same year another report on solid-state perovskite was published in science recording an
efficiency of 10.9% [19]. Since then, perovskite-based solar cell performance has rapidly
progressed with a best efficiency record of 22.1% in 2015 [6]. Progress in the perovskite
solar cells has been remarkable within a short time period and is considered as the biggest
scientific breakthrough in the PV industry [20–22].
Solution-based and vapor-based depositions are the two main deposition methods for
the fabrication of high quality perovskite thin films. The solution-based deposition tech-
nique is cost effective and compatible with the fabrication process, which includes flexible
substrates [23–25]. On the other hand, the vapor-based deposition technique is an indus-
trial production technique with a potential for the commercialization of perovskite solar
cells [26,27]. In either case, the deposition methods are relatively rapid and also consume
very small amount of materials. These are some of the reasons why scientific communities
are attracted to the perovskite solar cells industry.