Page 110 - Hybrid Enhanced Oil Recovery Using Smart Waterflooding
P. 110
102 Hybrid Enhanced Oil Recovery using Smart Waterflooding
(A)
1 1
KRW 0.5 KRO 0.5 0
0 -0.5
1 1
0.8 25 0.8 25
0.6 15 20 0.6 15 20
0.4 10 0.4 10
0.2 0 5 5
SW IFT(mN/m) SW 0.2 0 IFT(mN/m)
(B)
1 1
KRW 0.5 KRO 0.5 0
0 -0.5
1 1
0.8 25 0.8 20 25
0.6 15 20 0.6 10 15
0.4 5 10 0.4 5
SW 0.2 0 IFT(mN/m) SW 0.2 0 IFT(mN/m)
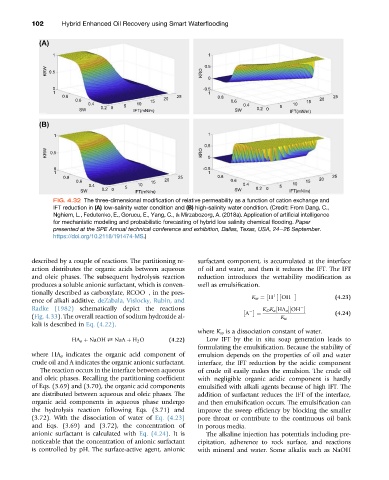
FIG. 4.32 The three-dimensional modification of relative permeability as a function of cation exchange and
IFT reduction in (A) low-salinity water condition and (B) high-salinity water condition. (Credit: From Dang, C.,
Nghiem, L., Fedutenko, E., Gorucu, E., Yang, C., & Mirzabozorg, A. (2018a). Application of artificial intelligence
for mechanistic modeling and probabilistic forecasting of hybrid low salinity chemical flooding. Paper
presented at the SPE Annual technical conference and exhibition, Dallas, Texas, USA, 24e26 September.
https://doi.org/10.2118/191474-MS.)
described by a couple of reactions. The partitioning re- surfactant component, is accumulated at the interface
action distributes the organic acids between aqueous of oil and water, and then it reduces the IFT. The IFT
and oleic phases. The subsequent hydrolysis reaction reduction introduces the wettability modification as
produces a soluble anionic surfactant, which is conven- well as emulsification.
tionally described as carboxylate, RCOO , in the pres-
K w ¼ H þ OH (4.23)
ence of alkali additive. deZabala, Vislocky, Rubin, and
Radke (1982) schematically depict the reactions K D K a ½HA o ½OH
A ¼ (4.24)
(Fig. 4.33). The overall reaction of sodium hydroxide al- K w
kali is described in Eq. (4.22).
where K w is a dissociation constant of water.
HA o þ NaOH % NaA þ H 2 O (4.22) Low IFT by the in situ soap generation leads to
formulating the emulsification. Because the stability of
where HA o indicates the organic acid component of emulsion depends on the properties of oil and water
crude oil and A indicates the organic anionic surfactant. interface, the IFT reduction by the acidic component
The reaction occurs in the interface between aqueous of crude oil easily makes the emulsion. The crude oil
and oleic phases. Recalling the partitioning coefficient with negligible organic acidic component is hardly
of Eqs. (3.69) and (3.70), the organic acid components emulsified with alkali agents because of high IFT. The
are distributed between aqueous and oleic phases. The addition of surfactant reduces the IFT of the interface,
organic acid components in aqueous phase undergo and then emulsification occurs. The emulsification can
the hydrolysis reaction following Eqs. (3.71) and improve the sweep efficiency by blocking the smaller
(3.72). With the dissociation of water of Eq. (4.23) pore throat or contribute to the continuous oil bank
and Eqs. (3.69) and (3.72), the concentration of in porous media.
anionic surfactant is calculated with Eq. (4.24).Itis The alkaline injection has potentials including pre-
noticeable that the concentration of anionic surfactant cipitation, adherence to rock surface, and reactions
is controlled by pH. The surface-active agent, anionic with mineral and water. Some alkalis such as NaOH