Page 111 - Hybrid Enhanced Oil Recovery Using Smart Waterflooding
P. 111
CHAPTER 4 Hybrid Chemical EOR Using Low-Salinity and Smart Waterflood 103
H 2 O 8
Na + A – ROCK 7
–
OH – M 6
HA o I
H 5
OIL
NaOH Interfacial Tension mN/m 4
HA o H 2 O 3
–
HA w A + H + 2
1
FIG. 4.33 The schematic description of reactions of
alkaline recovery process. (Credit: From deZabala, E. F., 0
0 1 2 3 4 5
Vislocky, J. M., Rubin, E., & Radke, C. J. (1982). A chemical
Chemical Concentration (wt %)
theory for linear alkaline flooding. SPE Journal, 22(02),
SDS NaOH
245e258. https://doi.org/10.2118/8997-PA.)
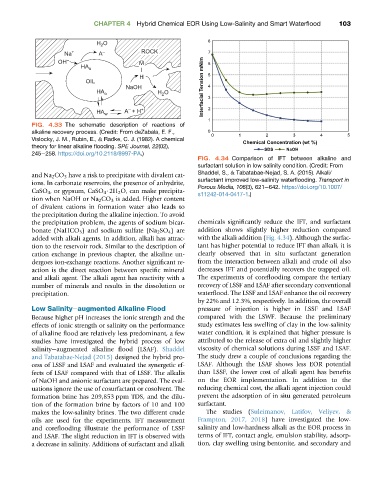
FIG. 4.34 Comparison of IFT between alkaline and
surfactant solution in low salinity condition. (Credit: From
and Na 2 CO 3 have a risk to precipitate with divalent cat- Shaddel, S., & Tabatabae-Nejad, S. A. (2015). Alkali/
surfactant improved low-salinity waterflooding. Transport in
ions. In carbonate reservoirs, the presence of anhydrite,
Porous Media, 106(3), 621e642. https://doi.org/10.1007/
CaSO 4 , or gypsum, CaSO 4 $2H 2 O, can make precipita-
s11242-014-0417-1.)
tion when NaOH or Na 2 CO 3 is added. Higher content
of divalent cations in formation water also leads to
the precipitation during the alkaline injection. To avoid
the precipitation problem, the agents of sodium bicar- chemicals significantly reduce the IFT, and surfactant
bonate (NaHCO 3 ) and sodium sulfate (Na 2 SO 4 ) are addition shows slightly higher reduction compared
added with alkali agents. In addition, alkali has attrac- with the alkali addition (Fig. 4.34). Although the surfac-
tion to the reservoir rock. Similar to the description of tant has higher potential to reduce IFT than alkali, it is
cation exchange in previous chapter, the alkaline un- clearly observed that in situ surfactant generation
dergoes ion-exchange reactions. Another significant re- from the interaction between alkali and crude oil also
action is the direct reaction between specific mineral decreases IFT and potentially recovers the trapped oil.
and alkali agent. The alkali agent has reactivity with a The experiments of coreflooding compare the tertiary
number of minerals and results in the dissolution or recovery of LSSF and LSAF after secondary conventional
precipitation. waterflood. The LSSF and LSAF enhance the oil recovery
by 22% and 12.3%, respectively. In addition, the overall
Low Salinityeaugmented Alkaline Flood pressure of injection is higher in LSSF and LSAF
Because higher pH increases the ionic strength and the compared with the LSWF. Because the preliminary
effects of ionic strength or salinity on the performance study estimates less swelling of clay in the low-salinity
of alkaline flood are relatively less predominant, a few water condition, it is explained that higher pressure is
studies have investigated the hybrid process of low attributed to the release of extra oil and slightly higher
salinityeaugmented alkaline flood (LSAF). Shaddel viscosity of chemical solutions during LSSF and LSAF.
and Tabatabae-Nejad (2015) designed the hybrid pro- The study drew a couple of conclusions regarding the
cess of LSSF and LSAF and evaluated the synergetic ef- LSAF. Although the LSAF shows less EOR potential
fects of LSAF compared with that of LSSF. The alkalis than LSSF, the lower cost of alkali agent has benefits
of NaOH and anionic surfactant are prepared. The eval- on the EOR implementation. In addition to the
uations ignore the use of cosurfactant or cosolvent. The reducing chemical cost, the alkali agent injection could
formation brine has 209,853 ppm TDS, and the dilu- prevent the adsorption of in situ generated petroleum
tion of the formation brine by factors of 10 and 100 surfactant.
makes the low-salinity brines. The two different crude The studies (Suleimanov, Latifov, Veliyev, &
oils are used for the experiments. IFT measurement Frampton, 2017, 2018) have investigated the low-
and coreflooding illustrate the performance of LSSF salinity and low-hardness alkali as the EOR process in
and LSAF. The slight reduction in IFT is observed with terms of IFT, contact angle, emulsion stability, adsorp-
a decrease in salinity. Additions of surfactant and alkali tion, clay swelling using bentonite, and secondary and