Page 60 - Hybrid Enhanced Oil Recovery Using Smart Waterflooding
P. 60
52 Hybrid Enhanced Oil Recovery using Smart Waterflooding
0.9
0.8
0.7
0.6
Oil Recovery 0.5 Live oil with HC interaction
0.4
Live oil without HC interaction
0.3 Dead oil with HC interaction
0.2
0.1
High Low High
Seawater salinity salinity salinity
0
0 0.5 1 1.5 2 2.5 3 3.5
PV
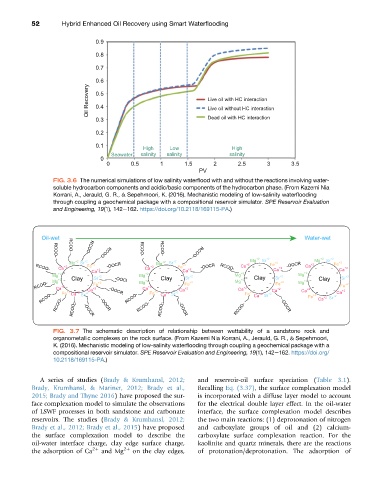
FIG. 3.6 The numerical simulations of low salinity waterflood with and without the reactions involving water-
soluble hydrocarbon components and acidic/basic components of the hydrocarbon phase. (From Kazemi Nia
Korrani, A., Jerauld, G. R., & Sepehrnoori, K. (2016). Mechanistic modeling of low-salinity waterflooding
through coupling a geochemical package with a compositional reservoir simulator. SPE Reservoir Evaluation
and Engineering, 19(1), 142e162. https://doi.org/10.2118/169115-PA.)
Oil-wet RCOO- RCOO- RCOO- Water-wet
RCOO-
RCOO-
+2 Sr +2 Mg +2 Sr +2
RCOO-
RCOO-
Mg
Mg +2 Sr +2 Mg +2 Sr +2 Fe +2 -OOCR Fe +2
+2 Fe +2 -OOCR +2 Fe +2 -OOCR RCOO- Ca +2 Ca +2
Ca +2 Ca +2 Ca +2 Ca +2
RCOO-
Ca Ca +2
Mg +2 +2 Mg +2 +2 Mg +2 +2 Mg +2
+2 Clay Sr -OOCI +2 Clay Sr Mg +2 Clay Sr +2 Clay Sr
RCOO- Mg Ca +2 Fe +2 Mg Ca +2 Fe +2 +2 Fe +2 Mg Fe +2
+2 +2 Sr +2 Ca +2 -OOCR Fe +2 Ca +2 Sr +2 Ca +2 Ca Fe +2 +2 +2 Ca +2 Ca +2 Ca +2
RCOO- RCOO- Ca Sr Fe +2 Ca +2 Sr +2
Fe Ca
RCOO- RCOO- -OOCR -OOCR RCOO- RCOO- -OOCR RCOO- -OOCR
FIG. 3.7 The schematic description of relationship between wettability of a sandstone rock and
organometallic complexes on the rock surface. (From Kazemi Nia Korrani, A., Jerauld, G. R., & Sepehrnoori,
K. (2016). Mechanistic modeling of low-salinity waterflooding through coupling a geochemical package with a
compositional reservoir simulator. SPE Reservoir Evaluation and Engineering, 19(1), 142e162. https://doi.org/
10.2118/169115-PA.)
A series of studies (Brady & Krumhansl, 2012; and reservoir-oil surface speciation (Table 3.1).
Brady, Krumhansl, & Mariner, 2012; Brady et al., Recalling Eq. (3.37), the surface complexation model
2015; Brady and Thyne 2016) have proposed the sur- is incorporated with a diffuse layer model to account
face complexation model to simulate the observations for the electrical double layer effect. In the oil-water
of LSWF processes in both sandstone and carbonate interface, the surface complexation model describes
reservoirs. The studies (Brady & Krumhansl, 2012; the two main reactions: (1) deprotonation of nitrogen
Brady et al., 2012; Brady et al., 2015) have proposed and carboxylate groups of oil and (2) calcium-
the surface complexation model to describe the carboxylate surface complexation reaction. For the
oil-water interface charge, clay edge surface charge, kaolinite and quartz minerals, there are the reactions
the adsorption of Ca 2þ and Mg 2þ on the clay edges, of protonation/deprotonation. The adsorption of