Page 63 - Hybrid Enhanced Oil Recovery Using Smart Waterflooding
P. 63
CHAPTER 3 Modeling of Low-Salinity and Smart Waterflood 55
(A)
High Sal (0.4 M) Low Sal (0.02 M)
15 15
pH 4 pH 7
pH 5 pH 8
Total Pressure (MPa) 5 Total Pressure (MPa) 5 0
pH 9
pH 6
10
10
0
-5 -5
0 5 101520 25 30 35 40 0 5 10 15 20 25 30 35 40
L (A) L (A)
(B) 1.0
BPS High
BPS Low
0.8
PTot at Peak, CC, High
0.6 PTot at Peak, CC, Low
Ptot at Peak, CP, High
BPS ((μmol/m 2 ) 2 ) or -P Tot (10 7 Pa) -0.2
0.4
PTot at Peak, CP, Low
0.2
0.0
-0.4
-0.6
-0.8
-1.0
-1.2
-1.4
4 5 6 7 8 9
pH
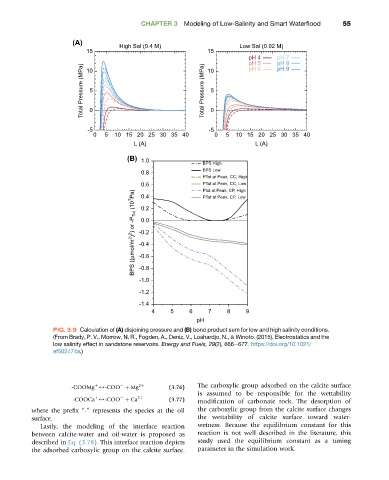
FIG. 3.9 Calculation of (A) disjoining pressure and (B) bond product sum for low and high salinity conditions.
(From Brady, P. V., Morrow, N. R., Fogden, A., Deniz, V., Loahardjo, N., & Winoto. (2015). Electrostatics and the
low salinity effect in sandstone reservoirs. Energy and Fuels, 29(2), 666e677. https://doi.org/10.1021/
ef502474a.)
þ
-COOMg 4-COO þ Mg 2þ (3.76) The carboxylic group adsorbed on the calcite surface
is assumed to be responsible for the wettability
-COOCa 4-COO þ Ca 2þ (3.77) modification of carbonate rock. The desorption of
þ
where the prefix “-” represents the species at the oil the carboxylic group from the calcite surface changes
surface. the wettability of calcite surface toward water-
Lastly, the modeling of the interface reaction wetness. Because the equilibrium constant for this
between calcite-water and oil-water is proposed as reaction is not well described in the literature, this
described in Eq. (3.78). This interface reaction depicts study used the equilibrium constant as a tuning
the adsorbed carboxylic group on the calcite surface. parameter in the simulation work.