Page 66 - Hybrid Enhanced Oil Recovery Using Smart Waterflooding
P. 66
58 Hybrid Enhanced Oil Recovery using Smart Waterflooding
Disjoining pressure Oil-wet
h∞
Oil Initial attractive force
Ψ1
h0 Brine θ Modified salinity
Ψ2 water injection
Disjoining pressure Water-wet Water film
Mineral Surface Final repulsive force
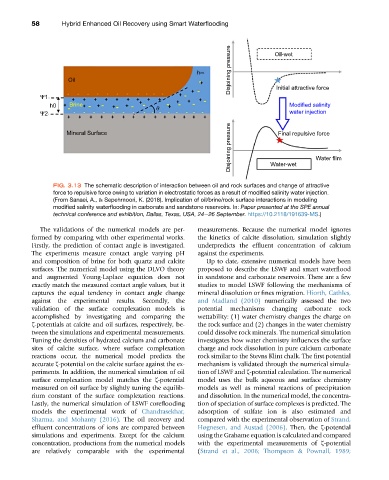
FIG. 3.13 The schematic description of interaction between oil and rock surfaces and change of attractive
force to repulsive force owing to variation in electrostatic forces as a result of modified salinity water injection.
(From Sanaei, A., & Sepehrnoori, K. (2018). Implication of oil/brine/rock surface interactions in modeling
modified salinity waterflooding in carbonate and sandstone reservoirs. In: Paper presented at the SPE annual
technical conference and exhibition, Dallas, Texas, USA, 24e26 September. https://10.2118/191639-MS.)
The validations of the numerical models are per- measurements. Because the numerical model ignores
formed by comparing with other experimental works. the kinetics of calcite dissolution, simulation slightly
Firstly, the prediction of contact angle is investigated. underpredicts the effluent concentration of calcium
The experiments measure contact angle varying pH against the experiments.
and composition of brine for both quartz and calcite Up to date, extensive numerical models have been
surfaces. The numerical model using the DLVO theory proposed to describe the LSWF and smart waterflood
and augmented Young-Laplace equation does not in sandstone and carbonate reservoirs. There are a few
exactly match the measured contact angle values, but it studies to model LSWF following the mechanisms of
captures the equal tendency in contact angle change mineral dissolution or fines migration. Hiorth, Cathles,
against the experimental results. Secondly, the and Madland (2010) numerically assessed the two
validation of the surface complexation models is potential mechanisms changing carbonate rock
accomplished by investigating and comparing the wettability: (1) water chemistry changes the charge on
z-potentials at calcite and oil surfaces, respectively, be- the rock surface and (2) changes in the water chemistry
tween the simulations and experimental measurements. could dissolve rock minerals. The numerical simulation
Tuning the densities of hydrated calcium and carbonate investigates how water chemistry influences the surface
sites of calcite surface, where surface complexation charge and rock dissolution in pure calcium carbonate
reactions occur, the numerical model predicts the rock similar to the Stevns Klint chalk. The first potential
accurate z-potential on the calcite surface against the ex- mechanism is validated through the numerical simula-
periments. In addition, the numerical simulation of oil tion of LSWF and z-potential calculation. The numerical
surface complexation model matches the z-potential model uses the bulk aqueous and surface chemistry
measured on oil surface by slightly tuning the equilib- models as well as mineral reactions of precipitation
rium constant of the surface complexation reactions. and dissolution. In the numerical model, the concentra-
Lastly, the numerical simulation of LSWF coreflooding tion of speciation of surface complexes is predicted. The
models the experimental work of Chandrasekhar, adsorption of sulfate ion is also estimated and
Sharma, and Mohanty (2016). The oil recovery and compared with the experimental observation of Strand,
effluent concentrations of ions are compared between Høgnesen, and Austad (2006). Then, the z-potential
simulations and experiments. Except for the calcium using the Grahame equation is calculated and compared
concentration, productions from the numerical models with the experimental measurements of z-potential
are relatively comparable with the experimental (Strand et al., 2006; Thompson & Pownall, 1989;