Page 128 - Adsorbents fundamentals and applications
P. 128
CARBON MOLECULAR SIEVES 113
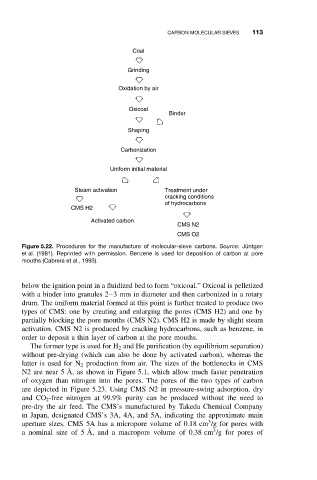
Coal
Grinding
Oxidation by air
Oxicoal
Binder
Shaping
Carbonization
Uniform initial material
Steam activation Treatment under
cracking conditions
of hydrocarbons
CMS H2
Activated carbon
CMS N2
CMS O2
Figure 5.22. Procedures for the manufacture of molecular-sieve carbons. Source:J ¨ untgen
et al. (1981). Reprinted with permission. Benzene is used for deposition of carbon at pore
mouths (Cabrera et al., 1993).
below the ignition point in a fluidized bed to form “oxicoal.” Oxicoal is pelletized
with a binder into granules 2–3 mm in diameter and then carbonized in a rotary
drum. The uniform material formed at this point is further treated to produce two
types of CMS: one by creating and enlarging the pores (CMS H2) and one by
partially blocking the pore mouths (CMS N2). CMS H2 is made by slight steam
activation. CMS N2 is produced by cracking hydrocarbons, such as benzene, in
order to deposit a thin layer of carbon at the pore mouths.
The former type is used for H 2 and He purification (by equilibrium separation)
without pre-drying (which can also be done by activated carbon), whereas the
latter is used for N 2 production from air. The sizes of the bottlenecks in CMS
N2 are near 5 ˚ A, as shown in Figure 5.1, which allow much faster penetration
of oxygen than nitrogen into the pores. The pores of the two types of carbon
are depicted in Figure 5.23. Using CMS N2 in pressure-swing adsorption, dry
and CO 2 -free nitrogen at 99.9% purity can be produced without the need to
pre-dry the air feed. The CMS’s manufactured by Takeda Chemical Company
in Japan, designated CMS’s 3A, 4A, and 5A, indicating the approximate main
3
aperture sizes. CMS 5A has a micropore volume of 0.18 cm /g for pores with
3
a nominal size of 5 ˚ A, and a macropore volume of 0.38 cm /g for pores of