Page 129 - Adsorbents fundamentals and applications
P. 129
114 ACTIVATED CARBON
Surface
(a) (b)
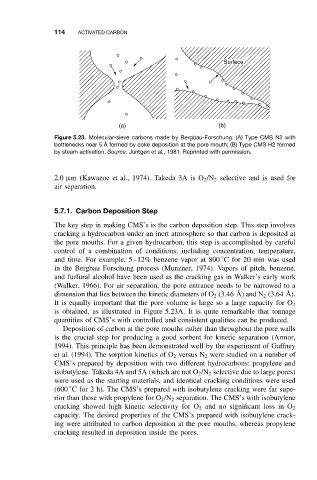
Figure 5.23. Molecular-sieve carbons made by Bergbau-Forschung: (A) Type CMS N2 with
bottlenecks near 5 ˚ A formed by coke deposition at the pore mouth; (B) Type CMS H2 formed
by steam activation. Source:J ¨ untgen et al., 1981. Reprinted with permission.
2.0 µm (Kawazoe et al., 1974). Takeda 3A is O 2 /N 2 selective and is used for
air separation.
5.7.1. Carbon Deposition Step
The key step in making CMS’s is the carbon deposition step. This step involves
cracking a hydrocarbon under an inert atmosphere so that carbon is deposited at
the pore mouths. For a given hydrocarbon, this step is accomplished by careful
control of a combination of conditions, including concentration, temperature,
◦
and time. For example, 5–12% benzene vapor at 800 C for 20 min was used
in the Bergbau Forschung process (Munzner, 1974). Vapors of pitch, benzene,
and furfural alcohol have been used as the cracking gas in Walker’s early work
(Walker, 1966). For air separation, the pore entrance needs to be narrowed to a
dimension that lies between the kinetic diameters of O 2 (3.46 ˚ A) and N 2 (3.64 ˚ A).
It is equally important that the pore volume is large so a large capacity for O 2
is obtained, as illustrated in Figure 5.23A. It is quite remarkable that tonnage
quantities of CMS’s with controlled and consistent qualities can be produced.
Deposition of carbon at the pore mouths rather than throughout the pore walls
is the crucial step for producing a good sorbent for kinetic separation (Armor,
1994). This principle has been demonstrated well by the experiment of Gaffney
et al. (1994). The sorption kinetics of O 2 versus N 2 were studied on a number of
CMS’s prepared by deposition with two different hydrocarbons: propylene and
isobutylene. Takeda 4A and 5A (which are not O 2 /N 2 selective due to large pores)
were used as the starting materials, and identical cracking conditions were used
◦
(600 C for 2 h). The CMS’s prepared with isobutylene cracking were far supe-
rior than those with propylene for O 2 /N 2 separation. The CMS’s with isobutylene
cracking showed high kinetic selectivity for O 2 and no significant loss in O 2
capacity. The desired properties of the CMS’s prepared with isobutylene crack-
ing were attributed to carbon deposition at the pore mouths, whereas propylene
cracking resulted in deposition inside the pores.