Page 133 - Adsorbents fundamentals and applications
P. 133
118 ACTIVATED CARBON
2
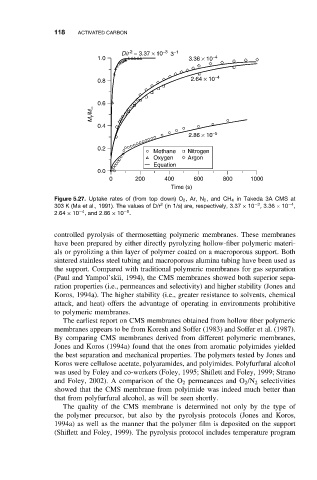
D/r = 3.37 × 10 −3 3 −1
1.0 3.36 × 10 −4
0.8 2.64 × 10 −4
0.6
M t /M ∞
0.4
2.86 × 10 −5
0.2
Methane Nitrogen
Oxygen Argon
Equation
0.0
0 200 400 600 800 1000
Time (s)
Figure 5.27. Uptake rates of (from top down) O 2 ,Ar, N 2 ,and CH 4 in Takeda 3A CMS at
−4
2
−3
303 K (Ma et al., 1991). The values of D/r (in 1/s) are, respectively, 3.37 × 10 ,3.36 × 10 ,
−5
−4
2.64 × 10 ,and 2.86 × 10 .
controlled pyrolysis of thermosetting polymeric membranes. These membranes
have been prepared by either directly pyrolyzing hollow-fiber polymeric materi-
als or pyrolizing a thin layer of polymer coated on a macroporous support. Both
sintered stainless steel tubing and macroporous alumina tubing have been used as
the support. Compared with traditional polymeric membranes for gas separation
(Paul and Yampol’skii, 1994), the CMS membranes showed both superior sepa-
ration properties (i.e., permeances and selectivity) and higher stability (Jones and
Koros, 1994a). The higher stability (i.e., greater resistance to solvents, chemical
attack, and heat) offers the advantage of operating in environments prohibitive
to polymeric membranes.
The earliest report on CMS membranes obtained from hollow fiber polymeric
membranes appears to be from Koresh and Soffer (1983) and Soffer et al. (1987).
By comparing CMS membranes derived from different polymeric membranes,
Jones and Koros (1994a) found that the ones from aromatic polyimides yielded
the best separation and mechanical properties. The polymers tested by Jones and
Koros were cellulose acetate, polyaramides, and polyimides. Polyfurfural alcohol
was used by Foley and co-workers (Foley, 1995; Shiflett and Foley, 1999; Strano
and Foley, 2002). A comparison of the O 2 permeances and O 2 /N 2 selectivities
showed that the CMS membrane from polyimide was indeed much better than
that from polyfurfural alcohol, as will be seen shortly.
The quality of the CMS membrane is determined not only by the type of
the polymer precursor, but also by the pyrolysis protocols (Jones and Koros,
1994a) as well as the manner that the polymer film is deposited on the support
(Shiflett and Foley, 1999). The pyrolysis protocol includes temperature program