Page 137 - Adsorbents fundamentals and applications
P. 137
122 ACTIVATED CARBON
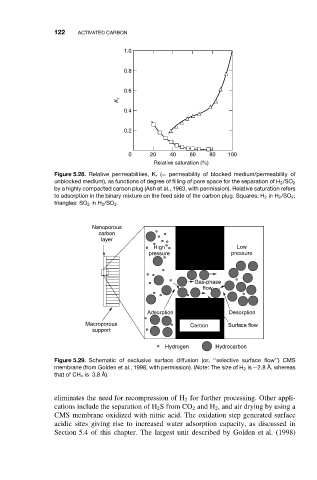
1.0
0.8
0.6
K r
0.4
0.2
0 20 40 60 80 100
Relative saturation (%)
Figure 5.28. Relative permeabilities, K r (= permeability of blocked medium/permeability of
unblocked medium), as functions of degree of filling of pore space for the separation of H 2 /SO 2
by a highly compacted carbon plug (Ash et al., 1963, with permission). Relative saturation refers
to adsorption in the binary mixture on the feed side of the carbon plug. Squares: H 2 in H 2 /SO 2 ;
triangles: SO 2 in H 2 /SO 2 .
Nanoporous
carbon
layer
High Low
pressure pressure
Gas-phase
flow
Adsorption Desorption
Macroporous Carbon Surface flow
support
Hydrogen Hydrocarbon
Figure 5.29. Schematic of exclusive surface diffusion (or, ‘‘selective surface flow’’) CMS
membrane (from Golden et al., 1998, with permission). (Note:The size of H 2 is ∼2.8 ˚ A, whereas
that of CH 4 is 3.8 ˚ A).
eliminates the need for recompression of H 2 for further processing. Other appli-
cations include the separation of H 2 S from CO 2 and H 2 , and air drying by using a
CMS membrane oxidized with nitric acid. The oxidation step generated surface
acidic sites giving rise to increased water adsorption capacity, as discussed in
Section 5.4 of this chapter. The largest unit described by Golden et al. (1998)