Page 135 - Adsorbents fundamentals and applications
P. 135
120 ACTIVATED CARBON
fluxes was obtained by exposure of the membranes to polypropylene (Jones and
Koros, 1994b). Jones and Koros (1995) also reported a solution to the problem
concerning the adverse effects of humidity by coating a thin layer of hydrophobic
polymer (e.g., Teflon) on the CMS membranes. Although small losses in selec-
tivity and productivity occur as a result of the resistance caused by adding the
polymer layer, the composite membranes are still very attractive when compared
with conventional polymer membranes (Jones and Koros, 1995).
An interesting method for coating the polymer layer on macroporous support
was described by Shiflett an Foley (1999). The polymer solution was sprayed on
the support with an ultrasonic nozzle, which generated droplets with relatively
uniform sizes and also minimized penetration into the support. A sintered stainless
steel tubing was used as the support, and a 25 wt % solution of polyfurfural alco-
hol (PFA) in acetone was sprayed through the nozzle. The ultrasonic nozzle was
operated at 40 kHz (the standard frequency for laboratory ultrasound cleaner).
◦
The deposited film was subsequently pyrolyzed at a heating rate of 5 C/min
◦
to 450 C for 2 h in He. SEM images showed the resulting membrane surface
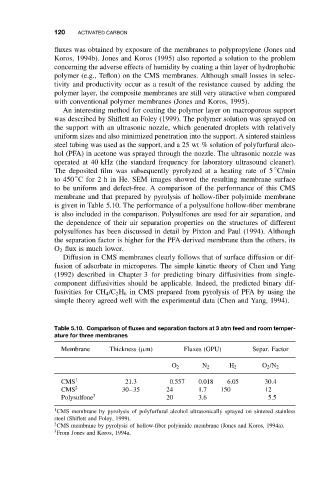
to be uniform and defect-free. A comparison of the performance of this CMS
membrane and that prepared by pyrolysis of hollow-fiber polyimide membrane
is given in Table 5.10. The performance of a polysulfone hollow-fiber membrane
is also included in the comparison. Polysulfones are used for air separation, and
the dependence of their air separation properties on the structures of different
polysulfones has been discussed in detail by Pixton and Paul (1994). Although
the separation factor is higher for the PFA-derived membrane than the others, its
O 2 fluxis muchlower.
Diffusion in CMS membranes clearly follows that of surface diffusion or dif-
fusion of adsorbate in micropores. The simple kinetic theory of Chen and Yang
(1992) described in Chapter 3 for predicting binary diffusivities from single-
component diffusivities should be applicable. Indeed, the predicted binary dif-
fusivities for CH 4 /C 2 H 6 in CMS prepared from pyrolysis of PFA by using the
simple theory agreed well with the experimental data (Chen and Yang, 1994).
Table 5.10. Comparison of fluxes and separation factors at 3 atm feed and room temper-
ature for three membranes
Membrane Thickness (µm) Fluxes (GPU) Separ. Factor
O 2 N 2 H 2 O 2 /N 2
CMS 1 21.3 0.557 0.018 6.05 30.4
CMS 2 30–35 24 1.7 150 12
Polysulfone 3 20 3.6 5.5
1 CMS membrane by pyrolysis of polyfurfural alcohol ultrasonically sprayed on sintered stainless
steel (Shiflett and Foley, 1999).
2 CMS membrane by pyrolysis of hollow-fiber polyimide membrane (Jones and Koros, 1994a).
3 From Jones and Koros, 1994a.