Page 226 - Adsorbents fundamentals and applications
P. 226
NATURE OF π-COMPLEXATION BONDING 211
− +
−
p* (2P*) C C
p (2P) C C + −
+ + −
Ag
+ −
− +
5S Ag Ag 4d yz
+
4d yz − + 4d z 2
(a) s-donation (b) d-p* backdonation (c) Redistribution
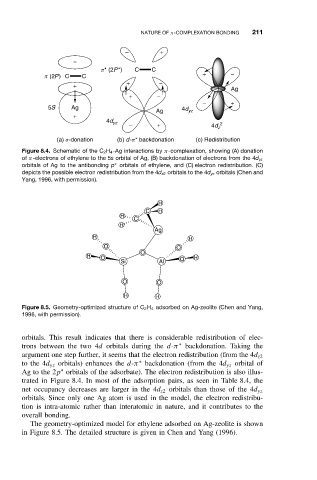
Figure 8.4. Schematic of the C 2 H 4 -Ag interactions by π-complexation, showing (A) donation
of π-electrons of ethylene to the 5s orbital of Ag, (B) backdonation of electrons from the 4d yz
∗
orbitals of Ag to the antibonding p orbitals of ethylene, and (C) electron redistribution. (C)
depicts the possible electron redistribution from the 4d z2 orbitals to the 4d yz orbitals (Chen and
Yang, 1996, with permission).
H
C H
H
C
H
Ag
H H
O O
O
H O H
Si Al O
O O
H H
Figure 8.5. Geometry-optimized structure of C 2 H 4 adsorbed on Ag-zeolite (Chen and Yang,
1996, with permission).
orbitals. This result indicates that there is considerable redistribution of elec-
trons between the two 4d orbitals during the d-π backdonation. Taking the
∗
argument one step further, it seems that the electron redistribution (from the 4d z2
∗
to the 4d yz orbitals) enhances the d-π backdonation (from the 4d yz orbital of
Ag to the 2p orbitals of the adsorbate). The electron redistribution is also illus-
∗
trated in Figure 8.4. In most of the adsorption pairs, as seen in Table 8.4, the
net occupancy decreases are larger in the 4d z2 orbitals than those of the 4d yz
orbitals. Since only one Ag atom is used in the model, the electron redistribu-
tion is intra-atomic rather than interatomic in nature, and it contributes to the
overall bonding.
The geometry-optimized model for ethylene adsorbed on Ag-zeolite is shown
in Figure 8.5. The detailed structure is given in Chen and Yang (1996).