Page 229 - Adsorbents fundamentals and applications
P. 229
214 π-COMPLEXATION SORBENTS AND APPLICATIONS
3.5
AgF
× 10 3 ] 2.5 AgCl
3
AgBr
Amount adsorbed [mmol/m 2 1.5
AgI
2
1
0.5
0
0 0.2 0.4 0.6 0.8 1
Partial pressure [atm]
◦
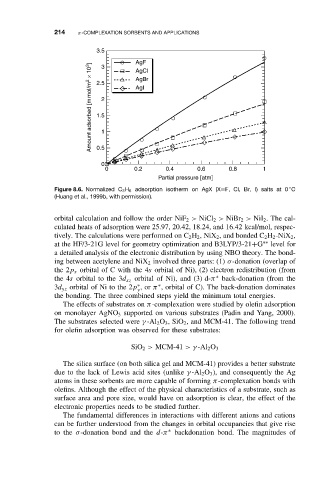
Figure 8.6. Normalized C 3 H 6 adsorption isotherm on AgX (X=F, Cl, Br, I) salts at 0 C
(Huang et al., 1999b, with permission).
orbital calculation and follow the order NiF 2 > NiCl 2 > NiBr 2 > NiI 2 . The cal-
culated heats of adsorption were 25.97, 20.42, 18.24, and 16.42 kcal/mol, respec-
tively. The calculations were performed on C 2 H 2 ,NiX 2 , and bonded C 2 H 2 -NiX 2 ,
at the HF/3-21G level for geometry optimization and B3LYP/3-21+G ∗∗ level for
a detailed analysis of the electronic distribution by using NBO theory. The bond-
ing between acetylene and NiX 2 involved three parts: (1) σ-donation (overlap of
the 2p x orbital of C with the 4s orbital of Ni), (2) electron redistribution (from
∗
the 4s orbital to the 3d xz orbital of Ni), and (3) d-π back-donation (from the
∗
∗
3d yz orbital of Ni to the 2p ,or π , orbital of C). The back-donation dominates
y
the bonding. The three combined steps yield the minimum total energies.
The effects of substrates on π-complexation were studied by olefin adsorption
on monolayer AgNO 3 supported on various substrates (Padin and Yang, 2000).
The substrates selected were γ -Al 2 O 3 ,SiO 2 , and MCM-41. The following trend
for olefin adsorption was observed for these substrates:
SiO 2 > MCM-41 >γ -Al 2 O 3
The silica surface (on both silica gel and MCM-41) provides a better substrate
due to the lack of Lewis acid sites (unlike γ -Al 2 O 3 ), and consequently the Ag
atoms in these sorbents are more capable of forming π-complexation bonds with
olefins. Although the effect of the physical characteristics of a substrate, such as
surface area and pore size, would have on adsorption is clear, the effect of the
electronic properties needs to be studied further.
The fundamental differences in interactions with different anions and cations
can be further understood from the changes in orbital occupancies that give rise
to the σ-donation bond and the d-π backdonation bond. The magnitudes of
∗