Page 234 - Adsorbents fundamentals and applications
P. 234
BULK SEPARATIONS BY π-COMPLEXATION 219
each bed to serve as the guard bed (Yang, 1987). Due to the steepness of the
CO isotherm, the adsorption pressure is unimportant. The desorption pressure is,
however, critically important. Generally, 0.1–0.3 atm pressure/vacuum is used for
desorption. Golden et al. (1998) described an in situ sorbent activation technique
+
for reducing Cu 2+ to the Cu state with the feed mixture (which was a synthesis
◦
gas) at 90 C. In all reports, the CO product purities were over 99 or 99.5%
with CO recoveries over 80%. With CuCl/γ -Al 2 O 3 ,theCH 4 impurity in the CO
product was 30 ppm (Golden et al., 1998).
8.4.3. Olefin/Paraffin Separations
Olefin/paraffin separations by PSA will be discussed in Chapter 10. In this chap-
ter, the isotherms of C 2 H 4 and C 3 H 6 on the best π-complexation sorbents will
be given. The sorbents discussed here should also be suitable for separations of
higher olefins from their corresponding paraffins.
Several new sorbents based on π-complexation have been prepared recently
+
for selective olefin adsorption (Yang et al., 2002). These include Ag -exchanged
resins (Yang and Kikkinides, 1995; Wu et al., 1999), monolayer CuCl/γ -Al 2 O 3
(Yang and Kikkinides, 1995), monolayer CuCl on pillared clays (Cheng and
Yang, 1995), monolayer AgNO 3 /SiO 2 (Rege et al., 1998; Padin and Yang, 2000),
and monolayer AgNO 3 supported on other substrates (Padin and Yang, 2000;
Yang et al., 2002), particularly on acid-treated clays (Cho et al., 2001; Choudary
et al., 2002). Among the different sorbents, monolayer AgNO 3 appears to give the
best results, while CuCl/γ -Al 2 O 3 appears to be the optimum when considering
the costs of the sorbents.
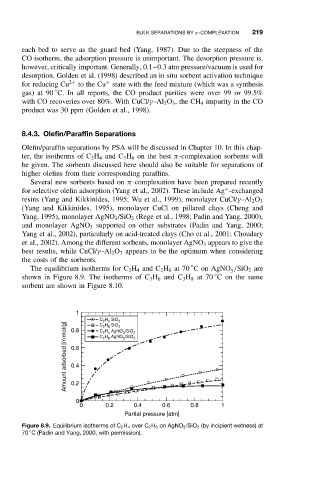
◦
The equilibrium isotherms for C 2 H 4 and C 2 H 6 at 70 ConAgNO /SiO 2 are
3
◦
shown in Figure 8.9. The isotherms of C 3 H 6 and C 3 H 8 at 70 Con thesame
sorbent are shown in Figure 8.10.
1
C H SiO 2 2 3 2
2 4
Amount adsorbed [m mol/g] 0.6 2 6 3 2
C H SiO
2 6
0.8
C H AgNO /SiO
2 4
C H AgNO /SiO
0.4
0.2
0
0 0.2 0.4 0.6 0.8 1
Partial pressure [atm]
Figure 8.9. Equilibrium isotherms of C 2 H 4 over C 2 H 6 on AgNO 3 /SiO 2 (by incipient wetness) at
◦
70 C (Padin and Yang, 2000; with permission).