Page 235 - Adsorbents fundamentals and applications
P. 235
220 π-COMPLEXATION SORBENTS AND APPLICATIONS
1.6
C H
3 6
1.4 C H
Amount adsorbed [m mol/g] 0.8
3 8
1.2
1
0.6
0.4
0.2
0
0 0.2 0.4 0.6 0.8 1
Partial pressure [atm]
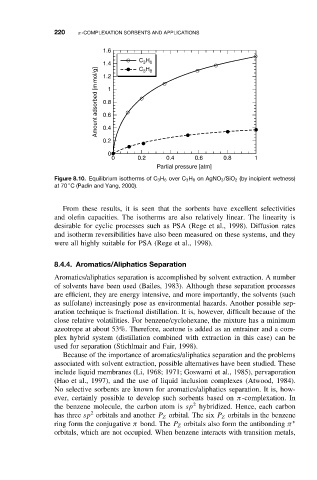
Figure 8.10. Equilibrium isotherms of C 3 H 6 over C 3 H 8 on AgNO 3 /SiO 2 (by incipient wetness)
◦
at 70 C (Padin and Yang, 2000).
From these results, it is seen that the sorbents have excellent selectivities
and olefin capacities. The isotherms are also relatively linear. The linearity is
desirable for cyclic processes such as PSA (Rege et al., 1998). Diffusion rates
and isotherm reversibilities have also been measured on these systems, and they
were all highly suitable for PSA (Rege et al., 1998).
8.4.4. Aromatics/Aliphatics Separation
Aromatics/aliphatics separation is accomplished by solvent extraction. A number
of solvents have been used (Bailes, 1983). Although these separation processes
are efficient, they are energy intensive, and more importantly, the solvents (such
as sulfolane) increasingly pose as environmental hazards. Another possible sep-
aration technique is fractional distillation. It is, however, difficult because of the
close relative volatilities. For benzene/cyclohexane, the mixture has a minimum
azeotrope at about 53%. Therefore, acetone is added as an entrainer and a com-
plex hybrid system (distillation combined with extraction in this case) can be
used for separation (Stichlmair and Fair, 1998).
Because of the importance of aromatics/aliphatics separation and the problems
associated with solvent extraction, possible alternatives have been studied. These
include liquid membranes (Li, 1968; 1971; Goswami et al., 1985), pervaporation
(Hao et al., 1997), and the use of liquid inclusion complexes (Atwood, 1984).
No selective sorbents are known for aromatics/aliphatics separation. It is, how-
ever, certainly possible to develop such sorbents based on π-complexation. In
2
the benzene molecule, the carbon atom is sp hybridized. Hence, each carbon
2
has three sp orbitals and another P Z orbital. The six P Z orbitals in the benzene
ring form the conjugative π bond. The P Z orbitals also form the antibonding π ∗
orbitals, which are not occupied. When benzene interacts with transition metals,