Page 236 - Adsorbents fundamentals and applications
P. 236
BULK SEPARATIONS BY π-COMPLEXATION 221
the π-orbitals of benzene can overlap with the empty outer-shell s orbital of
the transition metal to form a σ-bond. Moreover, it is possible that the vacant
∗
antibonding π -orbital of benzene can overlap with the d-orbitals in the transi-
tion metal similar to that formed in the olefin-Cu + bond (Huang et al., 1999).
Molecular orbital calculations indeed confirmed the π-complexation with benzene
(Takahashi et al., 2000).
Takahashi et al. (2000) studied π-complexation sorbents with benzene and
cyclohexane. Benzene and cyclohexane form an ideal pair of model compounds
for developing selective sorbents for aromatics. These molecules have similar
◦
◦
shapes and close boiling points (80 C for benzene and 81 C for cyclohex-
ane). The kinetic diameter of benzene, which is calculated from the minimum
equilibrium cross-sectional diameter, is estimated to be 5.85 ˚ A compared with
6.0 ˚ A for cyclohexane. The sorbents in that work were transition metal salts dis-
persed on high-surface-area substrates. Based on the results of selective olefin
+
+
4+
sorbents for olefin/paraffin separations, Cu ,Ag ,Pt ,and Pd 2+ cations were
the most promising sorbents due to their strong interactions with π-orbital to
olefin molecules. The sorbent that yielded the highest benzene selectivity was
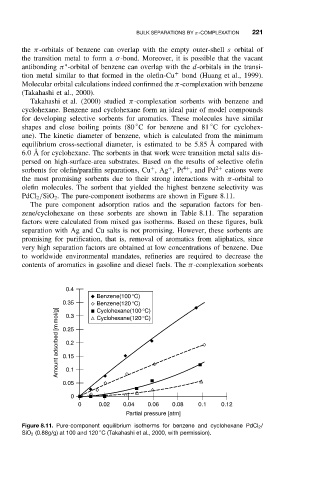
PdCl 2 /SiO 2 . The pure-component isotherms are shown in Figure 8.11.
The pure component adsorption ratios and the separation factors for ben-
zene/cyclohexane on these sorbents are shown in Table 8.11. The separation
factors were calculated from mixed gas isotherms. Based on these figures, bulk
separation with Ag and Cu salts is not promising. However, these sorbents are
promising for purification, that is, removal of aromatics from aliphatics, since
very high separation factors are obtained at low concentrations of benzene. Due
to worldwide environmental mandates, refineries are required to decrease the
contents of aromatics in gasoline and diesel fuels. The π-complexation sorbents
0.4
Benzene(100°C)
0.35 Benzene(120°C)
Amount adsorbed [m mol/g] 0.25
Cyclohexane(100°C)
0.3
Cyclohexane(120°C)
0.2
0.15
0.1
0.05
0
0 0.02 0.04 0.06 0.08 0.1 0.12
Partial pressure [atm]
Figure 8.11. Pure-component equilibrium isotherms for benzene and cyclohexane PdCl 2 /
◦
SiO 2 (0.88g/g) at 100 and 120 C (Takahashi et al., 2000, with permission).