Page 241 - Adsorbents fundamentals and applications
P. 241
226 π-COMPLEXATION SORBENTS AND APPLICATIONS
2
Amount adsorbed (m mol/g) 1.5
1
0.5
1,5-Hexadiene
1-Hexene
0
1.0E-05 1.0E-04 1.0E-03 1.0E-02 1.0E-01 1.0E+00
Partial pressure (atm)
◦
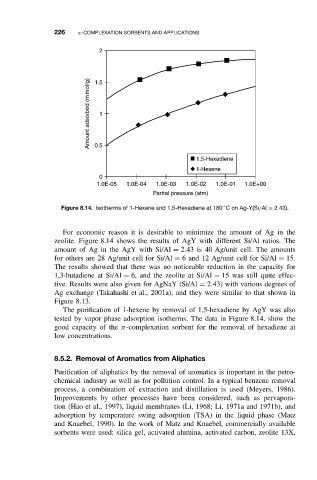
Figure 8.14. Isotherms of 1-Hexene and 1,5-Hexadiene at 180 C on Ag-Y(Si/Al = 2.43).
For economic reason it is desirable to minimize the amount of Ag in the
zeolite. Figure 8.14 shows the results of AgY with different Si/Al ratios. The
amount of Ag in the AgY with Si/Al = 2.43 is 40 Ag/unit cell. The amounts
for others are 28 Ag/unit cell for Si/Al = 6 and 12 Ag/unit cell for Si/Al = 15.
The results showed that there was no noticeable reduction in the capacity for
1,3-butadiene at Si/Al = 6, and the zeolite at Si/Al = 15 was still quite effec-
tive. Results were also given for AgNaY (Si/Al = 2.43) with various degrees of
Ag exchange (Takahashi et al., 2001a), and they were similar to that shown in
Figure 8.13.
The purification of 1-hexene by removal of 1,5-hexadiene by AgY was also
tested by vapor phase adsorption isotherms. The data in Figure 8.14. show the
good capacity of the π-complexation sorbent for the removal of hexadiene at
low concentrations.
8.5.2. Removal of Aromatics from Aliphatics
Purification of aliphatics by the removal of aromatics is important in the petro-
chemical industry as well as for pollution control. In a typical benzene removal
process, a combination of extraction and distillation is used (Meyers, 1986).
Improvements by other processes have been considered, such as pervapora-
tion (Hao et al., 1997), liquid membranes (Li, 1968; Li, 1971a and 1971b), and
adsorption by temperature swing adsorption (TSA) in the liquid phase (Matz
and Knaebel, 1990). In the work of Matz and Knaebel, commercially available
sorbents were used: silica gel, activated alumina, activated carbon, zeolite 13X,