Page 237 - Adsorbents fundamentals and applications
P. 237
222 π-COMPLEXATION SORBENTS AND APPLICATIONS
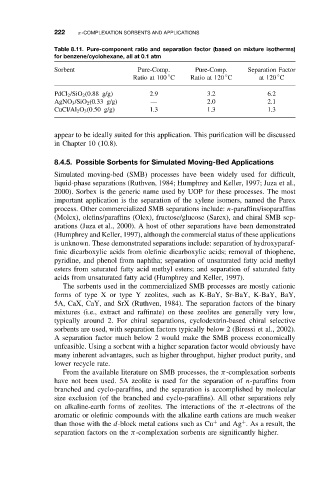
Table 8.11. Pure-component ratio and separation factor (based on mixture isotherms)
for benzene/cyclohexane, all at 0.1 atm
Sorbent Pure-Comp. Pure-Comp. Separation Factor
◦
◦
◦
Ratio at 100 C Ratio at 120 C at 120 C
PdCl 2 /SiO 2 (0.88 g/g) 2.9 3.2 6.2
AgNO 3 /SiO 2 (0.33 g/g) — 2.0 2.1
CuCl/Al 2 O 3 (0.50 g/g) 1.3 1.3 1.3
appear to be ideally suited for this application. This purification will be discussed
in Chapter 10 (10.8).
8.4.5. Possible Sorbents for Simulated Moving-Bed Applications
Simulated moving-bed (SMB) processes have been widely used for difficult,
liquid-phase separations (Ruthven, 1984; Humphrey and Keller, 1997; Juza et al.,
2000). Sorbex is the generic name used by UOP for these processes. The most
important application is the separation of the xylene isomers, named the Parex
process. Other commercialized SMB separations include: n-paraffins/isoparaffins
(Molex), olefins/paraffins (Olex), fructose/glucose (Sarex), and chiral SMB sep-
arations (Juza et al., 2000). A host of other separations have been demonstrated
(Humphrey and Keller, 1997), although the commercial status of these applications
is unknown. These demonstrated separations include: separation of hydroxyparaf-
finic dicarboxylic acids from olefinic dicarboxylic acids; removal of thiophene,
pyridine, and phenol from naphtha; separation of unsaturated fatty acid methyl
esters from saturated fatty acid methyl esters; and separation of saturated fatty
acids from unsaturated fatty acid (Humphrey and Keller, 1997).
The sorbents used in the commercialized SMB processes are mostly cationic
forms of type X or type Y zeolites, such as K-BaY, Sr-BaY, K-BaY, BaY,
5A, CaX, CaY, and SrX (Ruthven, 1984). The separation factors of the binary
mixtures (i.e., extract and raffinate) on these zeolites are generally very low,
typically around 2. For chiral separations, cyclodextrin-based chiral selective
sorbents are used, with separation factors typically below 2 (Biressi et al., 2002).
A separation factor much below 2 would make the SMB process economically
unfeasible. Using a sorbent with a higher separation factor would obviously have
many inherent advantages, such as higher throughput, higher product purity, and
lower recycle rate.
From the available literature on SMB processes, the π-complexation sorbents
have not been used. 5A zeolite is used for the separation of n-paraffins from
branched and cyclo-paraffins, and the separation is accomplished by molecular
size exclusion (of the branched and cyclo-paraffins). All other separations rely
on alkaline-earth forms of zeolites. The interactions of the π-electrons of the
aromatic or olefinic compounds with the alkaline earth cations are much weaker
+
+
than those with the d-block metal cations such as Cu and Ag . As a result, the
separation factors on the π-complexation sorbents are significantly higher.