Page 239 - Adsorbents fundamentals and applications
P. 239
224 π-COMPLEXATION SORBENTS AND APPLICATIONS
8.5.1. Removal of Dienes from Olefins
Normal α-olefins (NAO) are chemical intermediates used for making a variety of
products. The largest uses for NAOs are in the production of alcohols (via oxo
chemistry), as co-monomers for polyethylene production, and in the synthesis of
poly (α-olefins) for synthetic lubricants. Also, oligomerization of n-butenes to
more valuable octenes is an effective way of upgrading their value. A variety
of catalysts are used for these reactions. The most common metal-based catalyst
involves nickel, which is subject to poisoning by very low levels of 1,3-butadiene
(C 4 H 6 ). The selective butadiene hydrotreating process is one option to clean up
unwanted butadiene in a mixed-C 4 stream (Meyers, 1986). Removal of dienes
is also required for the production of higher α-olefins. Distillation is also being
used for these purification processes.
Sorbents based on π-complexation for olefin purification have been devel-
oped recently in the author’s laboratory (Padin et al., 1999; Jayaraman et al.,
2001; Padin et al., 2001; Takahashi et al., 2001a and 2001b). AgY and Cu(I)Y
are the best sorbents. Although only vapor phase isotherms are reported in the
literature cited above, these sorbents have been demonstrated successfully for
the liquid-phase feeds in the field. Diene impurities below 1 ppm can be readily
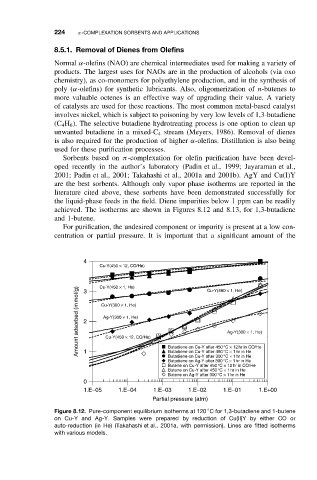
achieved. The isotherms are shown in Figures 8.12 and 8.13, for 1,3-butadiene
and 1-butene.
For purification, the undesired component or impurity is present at a low con-
centration or partial pressure. It is important that a significant amount of the
4
Cu-Y(450 × 12, CO/He) Cu-Y(450 × 1, He)
Cu-Y(450 × 1, He)
Amount adsorbed (mmol/g) 2 Cu-Y(300 × 1, He) Ag-Y(300 × 1, He)
3
Ag-Y(300 × 1, He)
Cu-Y(450 × 12, CO/He)
Butadiene on Cu-Y after 450°C × 12 hr in CO/He
1
Butadiene on Cu-Y after 450°C × 1hr in He
Butadiene on Cu-Y after 300°C × 1hr in He
Butadiene on Ag-Y after 300°C × 1hr in He
Butene on Cu-Y after 450°C × 12 hr in CO/He
Butene on Cu-Y after 450°C × 1hr in He
Butene on Ag-Y after 300°C × 1hr in He
0
1.E−05 1.E−04 1.E−03 1.E−02 1.E−01 1.E+00
Partial pressure (atm)
◦
Figure 8.12. Pure-component equilibrium isotherms at 120 C for 1,3-butadiene and 1-butene
on Cu-Y and Ag-Y. Samples were prepared by reduction of Cu(II)Y by either CO or
auto-reduction (in He) (Takahashi et al., 2001a, with permission). Lines are fitted isotherms
with various models.