Page 232 - Adsorbents fundamentals and applications
P. 232
BULK SEPARATIONS BY π-COMPLEXATION 217
3.5
Run 7 (AgY—reoxidized @ 350C 0.5atm 0.5h)
3 Run 8 (AgY—reoxidized @ 350C 0.13atm 0.5h)
Run 9 (AgY—reoxidized @ 400C 0.13atm 0.5h)
Amount adsorbed (m mol/g) 1.5 2 Run 2 (AgY—after H2 exposure @120C)
Run 10 (AgY—reoxidized @ 300 C 0.3atm 0.5h)
2.5
Run 1 (AgY—fresh sample degas @300C)
0.5 1
0
0.00001 0.0001 0.001 0.01 0.1 1
Partial pressure (atm)
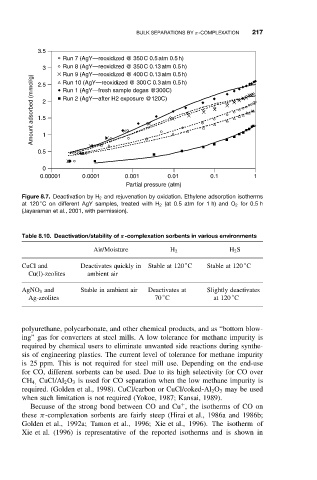
Figure 8.7. Deactivation by H 2 and rejuvenation by oxidation. Ethylene adsorption isotherms
◦
at 120 C on different AgY samples, treated with H 2 (at 0.5 atm for 1 h) and O 2 for 0.5 h
(Jayaraman et al., 2001, with permission).
Table 8.10. Deactivation/stability of π-complexation sorbents in various environments
Air/Moisture H 2 H 2 S
◦
◦
CuCl and Deactivates quickly in Stable at 120 C Stable at 120 C
Cu(I)-zeolites ambient air
AgNO 3 and Stable in ambient air Deactivates at Slightly deactivates
◦
◦
Ag-zeolites 70 C at 120 C
polyurethane, polycarbonate, and other chemical products, and as “bottom blow-
ing” gas for converters at steel mills. A low tolerance for methane impurity is
required by chemical users to eliminate unwanted side reactions during synthe-
sis of engineering plastics. The current level of tolerance for methane impurity
is 25 ppm. This is not required for steel mill use. Depending on the end-use
for CO, different sorbents can be used. Due to its high selectivity for CO over
CH 4, CuCl/Al 2 O 3 is used for CO separation when the low methane impurity is
required. (Golden et al., 1998). CuCl/carbon or CuCl/coked-Al 2 O 3 may be used
when such limitation is not required (Yokoe, 1987; Kansai, 1989).
+
Because of the strong bond between CO and Cu , the isotherms of CO on
these π-complexation sorbents are fairly steep (Hirai et al., 1986a and 1986b;
Golden et al., 1992a; Tamon et al., 1996; Xie et al., 1996). The isotherm of
Xie et al. (1996) is representative of the reported isotherms and is shown in