Page 228 - Adsorbents fundamentals and applications
P. 228
NATURE OF π-COMPLEXATION BONDING 213
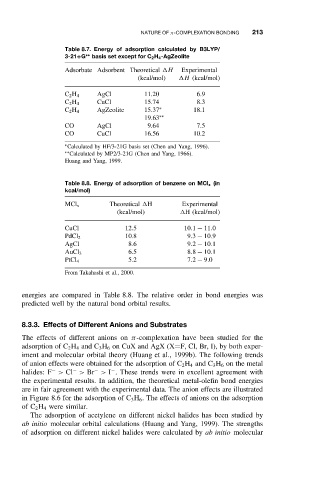
Table 8.7. Energy of adsorption calculated by B3LYP/
3-21+G ∗∗ basis set except for C 2 H 4 -AgZeolite
Adsorbate Adsorbent Theoretical H Experimental
(kcal/mol) H (kcal/mol)
AgCl 11.20 6.9
C 2 H 4
CuCl 15.74 8.3
C 2 H 4
AgZeolite 15.37 ∗ 18.1
C 2 H 4
19.63 ∗∗
CO AgCl 9.64 7.5
CO CuCl 16.56 10.2
Calculated by HF/3-21G basis set (Chen and Yang, 1996).
∗
Calculated by MP2/3-21G (Chen and Yang, 1966).
∗∗
Huang and Yang, 1999.
Table 8.8. Energy of adsorption of benzene on MCl x (in
kcal/mol)
MCl x Theoretical H Experimental
(kcal/mol) H (kcal/mol)
CuCl 12.5 10.1 − 11.0
10.8 9.3 − 10.9
PdCl 2
AgCl 8.6 9.2 − 10.1
6.5 8.8 − 10.1
AuCl 3
5.2 7.2 − 9.0
PtCl 4
From Takahashi et al., 2000.
energies are compared in Table 8.8. The relative order in bond energies was
predicted well by the natural bond orbital results.
8.3.3. Effects of Different Anions and Substrates
The effects of different anions on π-complexation have been studied for the
adsorption of C 2 H 4 and C 3 H 6 on CuX and AgX (X=F, Cl, Br, I), by both exper-
iment and molecular orbital theory (Huang et al., 1999b). The following trends
of anion effects were obtained for the adsorption of C 2 H 4 and C 3 H 6 on the metal
−
−
−
halides: F > Cl > Br > I . These trends were in excellent agreement with
−
the experimental results. In addition, the theoretical metal-olefin bond energies
are in fair agreement with the experimental data. The anion effects are illustrated
in Figure 8.6 for the adsorption of C 3 H 6 . The effects of anions on the adsorption
of C 2 H 4 were similar.
The adsorption of acetylene on different nickel halides has been studied by
ab initio molecular orbital calculations (Huang and Yang, 1999). The strengths
of adsorption on different nickel halides were calculated by ab initio molecular