Page 230 - Adsorbents fundamentals and applications
P. 230
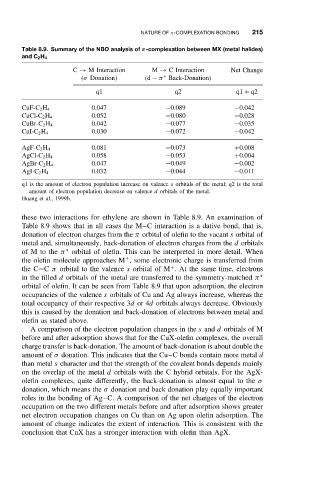
NATURE OF π-COMPLEXATION BONDING 215
Table 8.9. Summary of the NBO analysis of π-complexation between MX (metal halides)
and C 2 H 4
C → M Interaction M → C Interaction Net Change
(σ Donation) (d − π Back-Donation)
∗
q1 q2 q1 + q2
0.047 −0.089 −0.042
CuF-C 2 H 4
0.052 −0.080 −0.028
CuCl-C 2 H 4
0.042 −0.077 −0.035
CuBr-C 2 H 4
0.030 −0.072 −0.042
CuI-C 2 H 4
AgF-C 2 H 4 0.081 −0.073 +0.008
AgCl-C 2 H 4 0.058 −0.053 +0.004
AgBr-C 2 H 4 0.047 −0.049 −0.002
AgI-C 2 H 4 0.032 −0.044 −0.011
q1 is the amount of electron population increase on valence s orbitals of the metal; q2 is the total
amount of electron population decrease on valence d orbitals of the metal.
Huang et al., 1999b.
these two interactions for ethylene are shown in Table 8.9. An examination of
Table 8.9 shows that in all cases the M–C interaction is a dative bond, that is,
donation of electron charges from the π orbital of olefin to the vacant s orbital of
metal and, simultaneously, back-donation of electron charges from the d orbitals
of M to the π orbital of olefin. This can be interpreted in more detail. When
∗
the olefin molecule approaches M , some electronic charge is transferred from
+
+
the C=C π orbital to the valence s orbital of M . At the same time, electrons
in the filled d orbitals of the metal are transferred to the symmetry-matched π ∗
orbital of olefin. It can be seen from Table 8.9 that upon adsorption, the electron
occupancies of the valence s orbitals of Cu and Ag always increase, whereas the
total occupancy of their respective 3d or 4d orbitals always decrease. Obviously
this is caused by the donation and back-donation of electrons between metal and
olefin as stated above.
A comparison of the electron population changes in the s and d orbitals of M
before and after adsorption shows that for the CuX-olefin complexes, the overall
charge transfer is back-donation. The amount of back-donation is about double the
amount of σ donation. This indicates that the Cu–C bonds contain more metal d
than metal s character and that the strength of the covalent bonds depends mainly
on the overlap of the metal d orbitals with the C hybrid orbitals. For the AgX-
olefin complexes, quite differently, the back-donation is almost equal to the σ
donation, which means the σ donation and back donation play equally important
roles in the bonding of Ag–C. A comparison of the net changes of the electron
occupation on the two different metals before and after adsorption shows greater
net electron occupation changes on Cu than on Ag upon olefin adsorption. The
amount of change indicates the extent of interaction. This is consistent with the
conclusion that CuX has a stronger interaction with olefin than AgX.