Page 227 - Adsorbents fundamentals and applications
P. 227
212 π-COMPLEXATION SORBENTS AND APPLICATIONS
8.3.2. π-Complexation Bonds with Different Cations
The most important cations for π-complexation (for practical application) are
+
+
Ag and Cu ; hence they are used for comparison (Huang and Yang, 1999). It is
also interesting to compare the π-complexation between CO and ethylene, repre-
senting, respectively, C=Oand C=C bonds. The results of electron occupancies
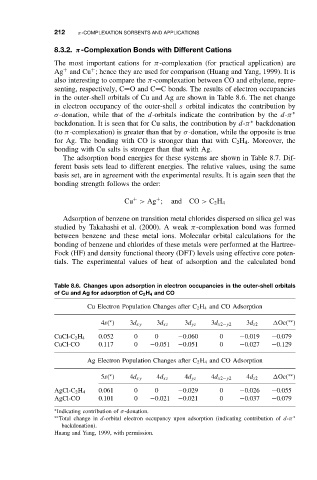
in the outer-shell orbitals of Cu and Ag are shown in Table 8.6. The net change
in electron occupancy of the outer-shell s orbital indicates the contribution by
σ-donation, while that of the d-orbitals indicate the contribution by the d-π ∗
∗
backdonation. It is seen that for Cu salts, the contribution by d-π backdonation
(to π-complexation) is greater than that by σ-donation, while the opposite is true
for Ag. The bonding with CO is stronger than that with C 2 H 4 . Moreover, the
bonding with Cu salts is stronger than that with Ag.
The adsorption bond energies for these systems are shown in Table 8.7. Dif-
ferent basis sets lead to different energies. The relative values, using the same
basis set, are in agreement with the experimental results. It is again seen that the
bonding strength follows the order:
+
+
Cu > Ag ; and CO > C 2 H 4
Adsorption of benzene on transition metal chlorides dispersed on silica gel was
studied by Takahashi et al. (2000). A weak π-complexation bond was formed
between benzene and these metal ions. Molecular orbital calculations for the
bonding of benzene and chlorides of these metals were performed at the Hartree-
Fock (HF) and density functional theory (DFT) levels using effective core poten-
tials. The experimental values of heat of adsorption and the calculated bond
Table 8.6. Changes upon adsorption in electron occupancies in the outer-shell orbitals
of Cu and Ag for adsorption of C 2 H 4 and CO
Cu Electron Population Changes after C 2 H 4 and CO Adsorption
∗∗
∗
4s( ) 3d xy 3d xz 3d yz 3d x2−y2 3d z2 Oc( )
CuCl-C 2 H 4 0.052 0 0 −0.060 0 −0.019 −0.079
CuCl-CO 0.117 0 −0.051 −0.051 0 −0.027 −0.129
Ag Electron Population Changes after C 2 H 4 and CO Adsorption
∗
∗∗
5s( ) 4d xy 4d xz 4d yz 4d x2−y2 4d z2 Oc( )
AgCl-C 2 H 4 0.061 0 0 −0.029 0 −0.026 −0.055
AgCl-CO 0.101 0 −0.021 −0.021 0 −0.037 −0.079
∗ Indicating contribution of σ-donation.
∗∗ Total change in d-orbital electron occupancy upon adsorption (indicating contribution of d-π ∗
backdonation).
Huang and Yang, 1999, with permission.