Page 233 - Adsorbents fundamentals and applications
P. 233
218 π-COMPLEXATION SORBENTS AND APPLICATIONS
60
a
50
Adsorption (ml/g adsorbent) 40 b
30
20
10
c
d
0 e
0 10 20 30 40 50 60 70 80
Pressure (kPa)
◦
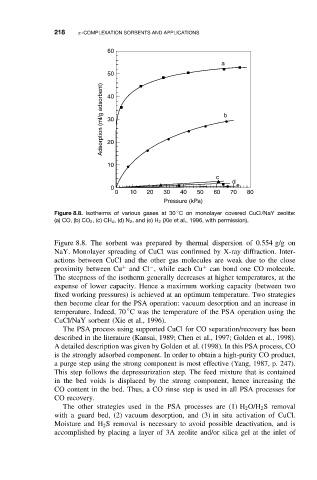
Figure 8.8. Isotherms of various gases at 30 C on monolayer covered CuCl/NaY zeolite:
(a) CO, (b) CO 2 ,(c) CH 4 ,(d) N 2 ,and (e)H 2 (Xie et al., 1996, with permission).
Figure 8.8. The sorbent was prepared by thermal dispersion of 0.554 g/g on
NaY. Monolayer spreading of CuCl was confirmed by X-ray diffraction. Inter-
actions between CuCl and the other gas molecules are weak due to the close
+
+
proximity between Cu and Cl , while each Cu can bond one CO molecule.
−
The steepness of the isotherm generally decreases at higher temperatures, at the
expense of lower capacity. Hence a maximum working capacity (between two
fixed working pressures) is achieved at an optimum temperature. Two strategies
then become clear for the PSA operation: vacuum desorption and an increase in
◦
temperature. Indeed, 70 C was the temperature of the PSA operation using the
CuCl/NaY sorbent (Xie et al., 1996).
The PSA process using supported CuCl for CO separation/recovery has been
described in the literature (Kansai, 1989; Chen et al., 1997; Golden et al., 1998).
A detailed description was given by Golden et al. (1998). In this PSA process, CO
is the strongly adsorbed component. In order to obtain a high-purity CO product,
a purge step using the strong component is most effective (Yang, 1987, p. 247).
This step follows the depressurization step. The feed mixture that is contained
in the bed voids is displaced by the strong component, hence increasing the
CO content in the bed. Thus, a CO rinse step is used in all PSA processes for
CO recovery.
The other strategies used in the PSA processes are (1) H 2 O/H 2 S removal
with a guard bed, (2) vacuum desorption, and (3) in situ activation of CuCl.
Moisture and H 2 S removal is necessary to avoid possible deactivation, and is
accomplished by placing a layer of 3A zeolite and/or silica gel at the inlet of