Page 286 - Adsorbents fundamentals and applications
P. 286
POLYMERIC RESINS 271
10 1
Wetted amberlite XAD-4
Amberlite XAD-7
XUS 43493.00
Ambersorb XE-563
Ambersorb XE-572
10 0 NORIT ROW 0.8 SUPRA
q/mol kg −1
10 −1
10 −2
10 −3 10 −2 10 −1 10 0 10 1 10 2
c/mol m −3
◦
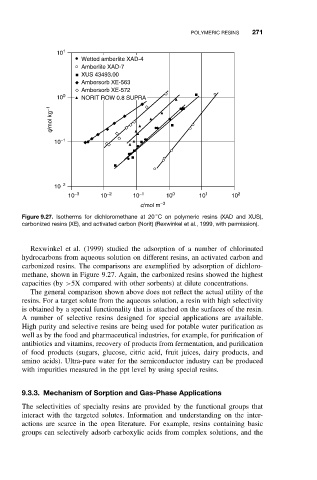
Figure 9.27. Isotherms for dichloromethane at 20 C on polymeric resins (XAD and XUS),
carbonized resins (XE), and activated carbon (Norit) (Rexwinkel et al., 1999, with permission).
Rexwinkel et al. (1999) studied the adsorption of a number of chlorinated
hydrocarbons from aqueous solution on different resins, an activated carbon and
carbonized resins. The comparisons are exemplified by adsorption of dichloro-
methane, shown in Figure 9.27. Again, the carbonized resins showed the highest
capacities (by >5X compared with other sorbents) at dilute concentrations.
The general comparison shown above does not reflect the actual utility of the
resins. For a target solute from the aqueous solution, a resin with high selectivity
is obtained by a special functionality that is attached on the surfaces of the resin.
A number of selective resins designed for special applications are available.
High purity and selective resins are being used for potable water purification as
well as by the food and pharmaceutical industries, for example, for purification of
antibiotics and vitamins, recovery of products from fermentation, and purification
of food products (sugars, glucose, citric acid, fruit juices, dairy products, and
amino acids). Ultra-pure water for the semiconductor industry can be produced
with impurities measured in the ppt level by using special resins.
9.3.3. Mechanism of Sorption and Gas-Phase Applications
The selectivities of specialty resins are provided by the functional groups that
interact with the targeted solutes. Information and understanding on the inter-
actions are scarce in the open literature. For example, resins containing basic
groups can selectively adsorb carboxylic acids from complex solutions, and the